sFile: <tabanidaekey.htm> <Medical
Index> <General Index> Site Description <Navigate to Home>
|
MYIASIS-CAUSING
ARTHROPODS KEY (Contact) ----Please
CLICK on desired highlighted categories to
view: Use Ctrl/F to search for subjects:
[Also
See: Key to Myiasis-causing Flies]
Service
(2008) considered different types of myiasis caused by Diptera as accidental,
obligatory or facultative. The accidental type comes about by
eating contaminated food containing eggs or larvae, which can lead to
discomfort in humans and more serious effects in animals. The obligatory type of myiasis requires that fly maggots live in the
host for all or part of their life cycle.
Facultative myiasis
results when larvae that are generally free-living also infect living
hosts. Throughout the literature
different terms are used to describe myiasis that affects different parts of
the body. Service (2008) gave the
following examples: Cutaneous = dermal or subdermal
myiasis; Urogenital myiasis; Ophthalmic = ocular myiasis; Nasopharyngeal myiasis; and Intestinal = gastrointestinal or
enteric myiasis. Referring to
appearance, there is Creeping Myiasis;
Furuncular Myiasis = boil-like
lesions occur; and Traumatic Myiasis
= when wounds are infested with living larvae. Matheson
(1950) noted that different names have been applied to the myiasis caused by
insect species in the different orders.
The term myiasis is
used for the Diptera that are responsible for the majority of cases, whereas
for Coleoptera it is canthariasis
and for Lepidoptera it is scoleciasis. The latter two types are comparatively
rare in humans. There are accidental cases of
myiasis involving Coleoptera, many of which are also of doubtful
validity. Matheson (1950) discusses
the larvae of Dermestidae and Tenebrionidae where infection might occur
through the consumption of cold cereals.
Most of these refer to Tenebrio
molitor, the mealworm, which is an important host of tapeworm,
Hymenolepis diminuta. Other beetle species that have been
associated with myiasis are Attagenus piceus
, Onthophagus bifasciatus,
O. unifasciatus, Caccobius mutans (see Caccobius
sp.), and Ptinus tectus. The larvae of
Lepidoptera have been connected with myiasis in a few rare cases, although
there is some doubt about their accuracy.
Matheson (1950) presented one more reliable case of a child who
consumed raw cabbage and later vomited larvae of the cabbage butterfly, Pieris brassicae. Church (1936) recorded a case where larvae
of the Corn Borer, Pyrausta nubilalis,
had attacked the body tissues of a woman. MYIASIS -- By Diptera There are
many authenticated cases of myiasis being caused by Diptera (flies). The Posterior Spiracular Plates
and Cephalopharyngeal Skeletons
of some Diptera larvae are used for identification (See: Key to
Myiasis-causing Flies. The following are arranged by separate
Diptera families: Sarcophagidae -- flesh Flies Sarcophagidae
adults are very abundant everywhere around decaying vegetation, animal matter
and excrement. Most species lay live
larvae and not eggs. Among the many
varied habits some species are parasitic on warm-blooded animals, on
grasshoppers and snails. Many are
scavengers and others are attracted to wounds. Because identification of larvae can be difficult it is best to
rear encountered larvae to the adult stage for proper identification. Larvae of the
genus Wohlfahrtia is frequently
involved in myiasis. One species, Wohlfahrtia vigil
Walker being unique among the Sarcophagidae by attacking healthy skin rather
than wounds or body orifices. Wohlfahrtia opaca Coq. of America and W. magnifica
Schiner of Europe are other important species in the genus but they
characteristically attack open wounds. The
cosmopolitan Sarcophaga haemorrhoidalis
Fall and other infecting species, S.
fuscicauda Keilin and S.
sarraceniae Riley,
are occasionally found important in causing myiasis particularly in the
intestinal tract.. Calliphoridae --
blowflies, bottleflies Callitroga americana (Cushing & Patton) is the screwworm fly is important
all over the Americas. It is an
obligate parasite of humans and animals that deposits larvae in open
wounds. Mortality rates are very high
among animals that it attacks, and humans can also perish if not treated
promptly. Callitroga macellaria (Fabr.), or secondary screwworm resembles C. americana, and
it is also widely distributed in the Americas. They usually deposit eggs on carrion but will also oviposit in
the wool of sheep and on wounds, and possibly on humans although this is
doubtful as there is confusion with C.
americana. Chrysomya bezziana is related to screwworm fly, but it is most common in
Africa, Asia and the Philippines. It
prefers to infest wounds of animals, but occasionally will attack humans
also. Other species of Chrysomya that occasionally attack
humans are C. marginalis
(Wied.) in Africa and C. albiceps
(Wied.), while in Europe, India and Africa, C.
chloropyga (Wied.) and C.
rufifacies (Macq.) are of some concern. Calliphora vomitoria (L.), C. vicina
R.-D. and C. livida Hall are common
bottle- or blowflies that cause myiasis by ovipositing in open wounds of
animals and occasionally humans. They
complete their life cycles from egg to adult in 2-4 weeks. In the genus Lucilia, which includes the green-bottle
flies, there are a number of species of medical importance because of their
involvement in myiasis. Included are C. americana (L.),
L. sericata Meig., L. illustris (Meig.),
L. cuprina (Wied.)
and L. silvarum (Meig.). Although myiasis has been attributed to
these flies under the species names noted, their identifications may be
inaccurate. Cordylobia anthropophaga (= Tumbu or Mango Fly) and Auchmeromyia
senegalensis (= Congo floor maggot) of Africa. Infestation occurs from contaminated
clothing that has not been washed or is placed on the ground to dry. The larvae of
Cordylobia anthropophaga
Grunberg, the tumbu fly of Africa, that begin their growth in decaying
organic matter, will penetrate the skin of animals and occasionally humans to
complete their development. Phormia regina (Meig.), the black blowfly, is a cosmopolitan species that
causes myiasis in animals and rarely in humans. Auchmeromyia luteola (Fab.), the Congo floor maggot, larvae attack humans by
feeding on their blood during the night.
They leave their hosts in daytime only to return again to feed at
night. Pollenia rudis (Fab.), the cluster fly, does not cause myiasis but annoys
people when adults enter dwellings.
In this case they are parasitic on earthworms. Musca domestica L., the common housefly, has caused intestinal myiasis in
humans. Infection can occur because
of this fly's close association with humans in their dwellings. Matheson (1950) details the many
situations where infection can occur, which are usually through a lack of
sanitation. Muscina stabulans (Fallen), the nonbiting stable fly, is sometimes abundant
around structures. Its habits are
similar to Musca domestica by
breeding in organic wastes, and it also has caused intestinal myiasis in
humans. Fanniidae (=Anthomyiidae) -- lesser
houseflies Species of
the genus Fannia often occur
together with Musca domestica
in the same habitats of decaying organic matter. Adults of Fannia canicularis (L.), the lesser
housefly, will occur in large numbers hovering in and outside of
structures. These flies are
particularly abundant where poultry dung provides an ideal breeding habitat. Fannia scalaris
Fab., the latrine fly, is smaller than F.
canicularis and with similar breeding habits. Matheson (1950) reports the existence of
numerous records where the larvae of these flies caused gastric and
intestinal myiasis in humans. Oestridae -- bot- & warble
flies This group of
flies is usually associated with domestic and wild animals, but there are
occasional cases of myiasis in humans.
Four subspecies are involved:
Oestrinae, Gastrophilinae, Hypodermatinae & Cuterebrinae. Two genera of particular medical importance
are Oestrus and Rhinoestrus. When humans come in close contact with sheep and other domestic
animals they may become infected (e.g., Oestrus
ovis L.). Adults of the
subfamily Cuterebrinae resemble
bees, and the larvae of all species are parasitic on mammals including
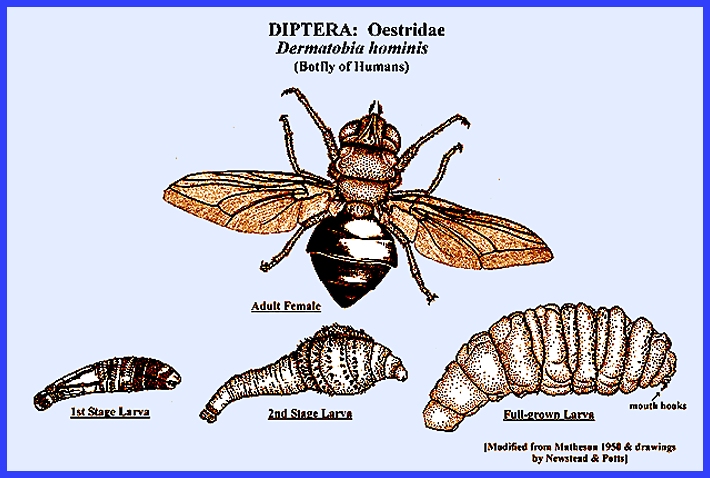
humans. Dermatobia
hominus.L, the human warble fly, is common especially in
forested regions of tropical America.
Females of this species infest other arthropods such as mosquitoes,
flies and ticks, with their eggs.
Humans become infected when coming into contact with the carriers. The warble fly eggs hatch when the carrier
contacts the host, and the larvae burrow into the skin, which is facilitated
by wounds caused by the carrier. One Cuterebra sp. has also been found to
attack humans. Hypoderma bovis L. & Hypoderma
lineatum (Villers) are the more common cosmopolitan warble
flies attacking cattle. However,
parasitism of humans is not uncommon, and infection is noticeable when the
larvae produce a swelling underneath the skin. Both Matheson (1950) and Service (2008) present detailed cases
of human infection and frequent encounters with Hypoderma
bovis. Botflies in
the subspecies Gastrophilinae resemble honeybees. Gasterophilus intestinalis,
G. haemorrhoidalis and G. nasalis are often found to attack humans. There are
many species in this Diptera family, all of which resemble bees. Tubifera
tenax (L.), the drone fly, is found hovering around rotting
organic matter upon which eggs are deposited. The larvae are distinctive because of a tail-like breathing
tube, giving them the name "rat-tailed maggots." There have been numerous cases of intestinal
myiasis recorded from humans with this species. Matheson (1950) stated that infection with these larvae would
probably have come from drinking fouled water containing young larvae or eggs
or ingesting rotting fruit. Other
less common species of Tubifera
that are suspected to have caused myiasis are T. argustorum and T.
dimidiatus. There are
also reported cases of intestinal myiasis caused by Syrphus species even though the group is
predacious on other insects.
Infection with this group is believed to result from ingesting
contaminated vegetables. On rare
occasions other Diptera species have been reported in cases of myiasis. Most often found are Psychoda albipennis and P. bipunctata Curt. (Psychodidae), Megaselia scalaris
(Phoridae), Piophila casei (Piophilidae), Rhyphus fenestralis Scop.
(Anisopodidae), Hermetia illucens L.
(Stratiomyidae). [See Matheson 1950 for more details]. In the 20th
Century the larvae of blowflies had been used in the treatment of
osteomyelitis by devouring dead and dying tissues and simultaneously
destroying any invading bacteria.
Thoroughly cleaned wounds hastened the healing process and the
larvae's saliva apparently possessed bactericidal properties. When myiasis
involves living larvae occurring in sores, wounds and dermal or sub dermal
tissues, their removal under aseptic conditions can be quite simple. However, when the larvae are deeper in the
tissues or when they have affected the mucous membranes, frontal sinuses or
eyes, removal can be complicated so that surgery may be required. In extreme cases the larvae may cause
major damage that cannot be undone. An accurate
diagnosis of the causative agents may be useful in the treatment of myiasis,
but infection should be precluded by a thorough knowledge of the environment
to avoid contaminated foods and the vector carriers of eggs or larvae
involving other arthropods such as mosquitoes and ticks. The use of insect repellants and proper
clothing can protect against contact.
Also there may be seasonal activity of vectors that can be avoided. - - - - - - - - - - - - - - - - - - - - Key References: <medvet.ref.htm> <Hexapoda> Abram, L. J. & A. I. Froimson. 1987.
Myiasis (maggot infection) as a complication of fracture management: a
case report and rev.of literature. Orthopedics 10: 625-27. Aldrich, J. M. 1916.
Sarcophaga and allies in
North America. Thomas Say Found. Ent.
Soc. Amer. Andre, Emile. 1925. Sur un cas de
myiase cutanee chez l'homme.
Parasitology 17: 173-75. Arbit, E., R. E. Varon & S. S.
Brem. 1986. Myiatic scalp and skull infection with
Diptera Sarcophaga: a case
report. Neurosurgery
18: 361-62 Austen, E. E. 1912. British flies which cause myiasis in man. Rept. Local Govt. Bd. Pub. Hlth & Med.
66: 5-15 Baer, W. S. 1931.
The treatment of chronic osteomyelitis with the maggot (larva of
blow-fly). J. Bone & Joint Surg.
13: 438-475. Beckendorf, R., S. A. Klotz, N. Hinkle
& W. Bartholomew. 2002. Nasal myiasis in an intensive care unit
linked to hospital-wide mouse infestation.
Archives Internal Medicine 162:
638-640. Brand, A. F. 19031.
Gastro-intestinal myiasis.
1931. A report of a case. Arch.
Internal. Med. 47: 149-154. Catts, E. P. 1982.
Biology of New World bot flies:
Cuterebridae. Ann. Rev. Ent.
27: 313-38. Colwell, D. D., M. J. R. Hall & P. J. Scholl
(eds.) 2006. The Oestrid Flies:
Biology, Host-Parasite Relationships, Impact & Management. CABI, Wallingford. Dixon, O. J. 1924.
An unusual case of rhinal myiasis with recovery. J. Amer. Med. Assoc. 83: 1332-1333. Dove, W. E. 1937.
Myiasis of man. J. Econ. Ent. 30: 29-39. Gabaj, M. M., A. M. Gusbi & M. A. Q. Awan. 1989. First human infestations in Africa with larvae of the American
screwworm, Cochliomyia hominivorax Coq.
Ann. Trop. Med. & Parasitol. 83:
553-54. Graham, O. H. (ed.) 1985. Symposium on eradication of the
screwworm from the United States & Mexico. Misc. Publ. Ent. Soc. Amer. 62: 1-68. Greenberg, B. &
J. C. Kunich. 2002. Entomology and the Law: Flies as Forensic
Indicators. Cambridge Univ. Press. Hall, M. J. R. & R.
Wall. 1995. Myiasis of humans and domestic animals. Adv. Parasitol. 35: 257-334. Hinman, F. H. & E.
C. Faust. 1932. The ingestion of the larvae of Tenebrio molitor L. (meal worm) by
man. J. Parasit. 19: 119-20. Hope, F. W. 1840.
On insects and their larvae in the human body. Trans. Ent. Soc. London 2: 256-271. Kersten, R., N. M.
Shoukrey & K. F. Tabbara.
1986. Orbital myiasis. Ophthalmology 93: 1228-1232. Krafsur, E. S., C. J.
Whitten & J. E. Novy. 1987. Screwworm eradication in North and Central
America. Parasitology Today 3: 131-137. Lane, R. P., C. R. Lovell, W. A. D. Griffiths &
T. S. Sonnex. 1987. Human cutaneous myiasis: a review and
report of three cases due to Dermatobia
hominis. Clinical &
Exptal. Dermatol. 12: 40-45. Legner, E. F. 1995.
Biological control of Diptera of medical and veterinary
importance. J. Vector Ecology 20(1):
59-120. Legner, E. F.. 2000.
Biological control of aquatic Diptera. p. 847_870.
Contributions to a Manual of Palaearctic Diptera, Vol. 1, Sci. Herald, Budapest. 978 p. Liggett, H. 1931.
Parasitic invasion of the nose.
J. Amer. Med. Assoc. 96:
1571-72. Lindner, E., 1930. 1a. Phryneidae (Anisopodidae, Rhyphidae). In: E. Lindner, Die Fliegen der palaearktischen Region,
vol. 2, pt. 1. Stuttgart, 10 pp. Matheson, R.
1950. Medical Entomology. Comstock Publ. Co, Inc. 610 p. Meillon, B. de. 1937. A note
on two beetles of medical interest in Natal.
S. Afr. Med. J. p. 479. Meleny, H. E. & P. D. Harwood. Human intestinal myiasis due to the larvae
of the soldier fly, Hermetia illucens
Linne. Amer. J. Trop. Med. 15: 45-49. Nunzi, E., F.
Rongioletti & A. Rebora.
1986. Removal of Dermatobia hominis larvae. Archives of Dermatol. 122:
140. Palmer, E. D. 1946. Intestinal
canthariasis due to Tenebrio molitor. J. Parasitol. 32: 54-55. Service, M. 2008. Medical
Entomology For Students. Cambridge
Univ. Press. 289 p Sharpe, D. S. 1947.
An unusual case of intestinal myiasis. British Med. J. 1: 54. Sherman, R. A., M. J. R. Hall & S. Thomas. 2000.
Medicinal maggots: an ancient remedy for some contemporary
afflictions. Ann. Rev. Ent. 45: 55-61. Shoaib, K. A. A., P. J. McCall, R. Goyal, S.
Loganathan & W. D. Richmond.
2000. First urological presentation
of new world screw worm (Cochliomyia
hominovorax) myiasis in the
United Kingdom: a case report.
British J. Urology-International 86:
16-17. Smith, K. G. V. 1986.
A Manual of Forensic Entomology.
British Mus. Nat. Hist. & Cornell Univ. Press pp.
93-137. Spradbery, J. P. 1991.
A Manual for the Diagnosis of Screw-Worm Fly. CSIRO, Div. of Entomology. Zumpt, F. 1965.
Myiasis in Man and Animals in the Old World: a Textbook for
Physicians, Veterinarians and Zoologists.
Butterworth Publ., London. |