|
Arthropoda: Insecta
KEY TO SIPHONAPTERA
OF
MEDICAL IMPORTANCE
(Fleas)
(Contact)
Please CLICK on picture and
underlined links to view or to navigate within the key:
To Search for Subject Matter use Ctrl/F
[Also See: Siphonaptera Details]
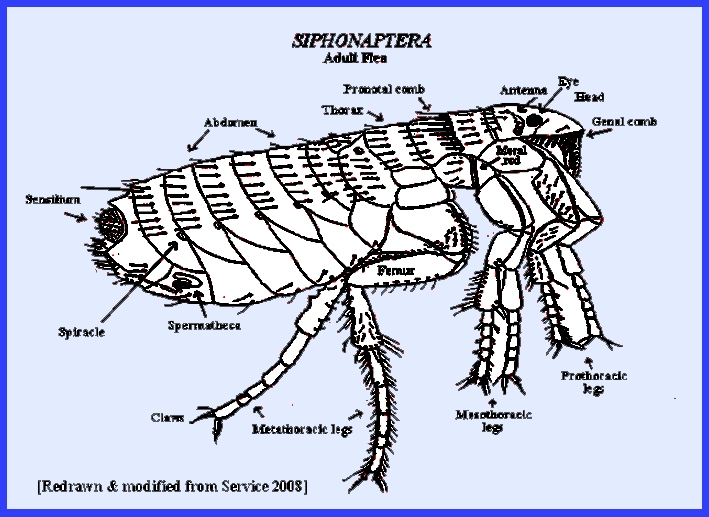 There are about 221 genera and over 2,205 species of fleas in
the world. The order has five
families with species of medical importance:
Hectopsyllidae, Dolichopsyllidae, Pulicidae, Hystrichopsyllidae and
Ischnopsyllidae. Thirteen medically
important species are: Ctenocephalides
canis (Curtis) [dog flea], Ctenocephalides felis
(Bouche) [cat flea], Cediopsylla simplex
(Baker) [rabbit flea], Ceratophyllus gallinae
(Schrank) [chicken or hen flea], Ctenophthalmus pseudargyrtes
Baker [Small mammal flea], Echidnophaga gallinacea (Westwood) [stick tight flea], Hoplopsyllus anomalus Baker [rodent flea], Leptopsylla segnis [European
mouse flea], Nosopsyllus fasciatus
(Bosc.) [rat flea], Oropsylla montana (Baker)
[ground squirrel flea], Pulex irritans L. [flea of humans], Tunga penetrans L.
[jigger flea] and Xenopsylla cheopis
(Roth.) [Oriental rat flea]. The
common names of fleas (e.g. "dog flea") are misleading as humans
may also be attacked by any of these species especially when in close
proximity of the preferred host. New
discoveries of medically important species are being made in South America;
e.g., Ectinorus insignis (Beaucournu
et al 2013) and Ctenidiosomus sp. (Lopez-Berrizbeitia et al. 2015). There are about 221 genera and over 2,205 species of fleas in
the world. The order has five
families with species of medical importance:
Hectopsyllidae, Dolichopsyllidae, Pulicidae, Hystrichopsyllidae and
Ischnopsyllidae. Thirteen medically
important species are: Ctenocephalides
canis (Curtis) [dog flea], Ctenocephalides felis
(Bouche) [cat flea], Cediopsylla simplex
(Baker) [rabbit flea], Ceratophyllus gallinae
(Schrank) [chicken or hen flea], Ctenophthalmus pseudargyrtes
Baker [Small mammal flea], Echidnophaga gallinacea (Westwood) [stick tight flea], Hoplopsyllus anomalus Baker [rodent flea], Leptopsylla segnis [European
mouse flea], Nosopsyllus fasciatus
(Bosc.) [rat flea], Oropsylla montana (Baker)
[ground squirrel flea], Pulex irritans L. [flea of humans], Tunga penetrans L.
[jigger flea] and Xenopsylla cheopis
(Roth.) [Oriental rat flea]. The
common names of fleas (e.g. "dog flea") are misleading as humans
may also be attacked by any of these species especially when in close
proximity of the preferred host. New
discoveries of medically important species are being made in South America;
e.g., Ectinorus insignis (Beaucournu
et al 2013) and Ctenidiosomus sp. (Lopez-Berrizbeitia et al. 2015).
The following keys separate the most common Genera and Species involved:
[Please CLICK on Figures to view]
|
KEY TO PRINCIPAL IMPORTANT GENERA
1a. Combs and
Meral Rods on thorax are present (Fig. A, Fig. B, Fig. C) - - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - - - 2a
No combs are present on thorax (Fig. D, Fig. E, Fig. F) - -- - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4a
2a. Meral rod on
lateral thorax is vertical (Fig. C)- - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Nosopsyllus
spp. (species)
Meral rod is angled (Fig. A, Fig. B) - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - - - - 3a
3a. Meral rod on
lateral thorax is strongly angled (Fig. B)- - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - Leptopsylla spp.
(species)
Meral rod is not as strongly angled (Fig. A) - - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - Ctenocephalides
spp. (species)
4a. Meral rod is
present on lateral thorax (Fig. F) -
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
- - - Xenopsylla spp. (species)
No meral rod is present on lateral
thorax (Fig. D,
Fig. E) - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - - - 5a
5a. Antenna on head
extends beyond an eye and first three thoracic segments about equal in size
(Fig. D) - - - - - - - Pulex spp. (species)
Antenna is shorter, not extending
beyond eye and first three thoracic segments are unequal in size (Fig. E) - - - Tunga
spp. (species)
KEY TO PRINCIPAL IMPORTANT SPECIES
1. A greatly reduced
thorax, with terga combined being shorter than the 1st abdominal
tergum. Gravid females are greatly
distended
(Fig. 1) _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ Hectopsyllidae
The
thorax is not reduced and the combined terga are usually longer than the
first abdominal tergum (Fig.
2) _ _ _ _ _ _ _ _ _ _ _ _ 3
2. Third leg coxa with a
patch of small spines on the inner surface. Abdominal segments 2 & 3 have spiracles (Fig. 3) _
_ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Echidnophaga gallinacea
Coxa of
3rd leg lacks the small spines.
Segments 2 & 3 of female abdomen lack spiracles. Species confined to warmer climates _ _ _
_ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Tunga penetrans
3. Terga of abdomen
usually with only one cross row of setae (Fig. 4). A groove between the frons and occiput
is usually absent. Eyes are
usually present _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ Pulicidae 4
Abdominal terga usually with more than one cross row of setae; There
is usually a groove between the frons and occiput. _ _ _ _ _ 9
4. A genal comb, or
crenidium is absent (Fig.
5c) _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _5
Genal and
pronotal combs are present (Fig. 5a) _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _7
5. Pronotal comb or
ctenidium is absent (Fig.
5c) _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ 6
Pronotal comb is present. Flea usually found on ground squirrels (Fig. 5g) _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Hoplopsyllus anomalus
6. Thorax second segment
pleuron divided by a stout, vertical rod like thickening _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Xenopsylla cheopis
Pleuron
is not divided _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Pulex irritans
7. Genal comb teeth are
straight, blunt and with black spines arranged almost vertically (Fig. 5i). usually found on rabbits in North
America
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Cediopsylla simplex
Genal
comb teeth hve 7-8 sharp teeth, and are arranged almost parallel to the
flea's long axis (Fig.
5a) _ Ctenocephalides spp. 8
8. Frons is high & rounded. First 2 spines of genal comb shorter
than the remaining (Fig.
5b) _ _ _ _ _ _ _ _ Ctenocephaldes canis
Frons
is low, flat and almost pointed.
Spines of the genal comb are about of equal length (Fig. 5a) _ _ _ _ _
_ Ctenocephalides felis
9. The head is a bit
elongated and 2-3 ventral flaps are present on each side near the fronto
genal angle (Fig. 5i). These fleas are
parasites of bats. _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Ischnopsyllidae
Head is
not elongated and there are no ventral flaps. Fleas are not bat parasites
_ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ 10
10. Genal comb absent, but combs on abdominal terga often present. Hexactenus ischnopsyllus _ _ _ Dolichopsyllidae 11
The
genal comb is present, but the combs on the abdominal terga are often
present _ _ _ _ _ _ _ _ _ _ _ Hystrichopsyllidae 12
11. The pronotal comb has
12 or more spines on each side _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _Ceratophyllus gallinae
Pronotal comp has less than 12 spines on each side (Fig. 5d). On male the finger of clasper is short,
broad, flattened and has spines
but
no black spinifrons _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _Nosopsyllus fasciatus
Movable finger of the clasper elongated &
sword-shaped. On ground squirrels
that carry plague (Fig.
5e) _Oropsylla montana
12. Genal comb contains 3 sharp
teeth directed backward. Usually
found on small rodents _ _ _ _Ctenophthalmus pseudargyrtes
Genal
comb has 4 blunt teeth directed backwards. (Fig. 5h). Common on rodents _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Leptopsylla segnis
|
- - - - - - - - - - - - - -
- - - - - -
Key References: <medvet.ref.htm> <Hexapoda>
Azad, A. F. 1990.
Epidemiology of murine typhus.
Ann. Rev. Ent. 35: 553-69.
Azad, A. F. & C. B.
Beard. 1998. Rickettsial pathogens and their arthropod
vectors. Emerging Infectious Diseases
4: 179-86.
Beaucournu, J-C. 2013. A new flea, Ectinorus
insignis n. sp. (Siphonaptera, Rhopalopsyllidae, Parapsyllinae),
with notes on the subgenus Ectinorus
in Chile and comments on unciform sclerotization
in the superfamily Malacopsylloidea. Parasite 20(35).
Bishopp, F. C. 1931.
Fleas and their control. U.S.
Dept. Agr., Farmers' Bull. 897.
Eisle, M. , J. Heukelbach &
van Marck, E. et al. 2003. Investigations on the biology, edpidemiology, pathology and
control of tunga penetrans in
Brazil:
I. Natural history in man. Parasitology Res. 49: 557-65.
Ewing, H. E. 1924.
Notes on the taxonomy and natural relationships of fleas, with
descriptions of four new species.
Parasitology 16: 341-254.
Ewing, H. E. & I.
Fox. 1943. The fleas of North America.
U.S. Dept. Agr. Misc. Pub. 500.
Fox, Irving. 1940.
Fleas of the eastern United States.
Ames, Iowa Press
Gage, K. L. & Y.
Kosoy. 2005. Natural history of plague: perspectives
from more than a century of research.
Ann. Rev. Ent. 50: 505-28.
Gratz, N. G. 1999.
Control of plague transmission.
IN: Plague Manual: Epidemiology, Distribution, Surveillance &
Control. WHO, Geneva. pp. 97-
134
Hechemy, K. E. & A. F. Azad. 2001. Endemic
typhus, and epidemic typhus. IN: The Encyclopedia of Arthropod-Transmitted
Infections of Man and
Domesticated Animals. CABI, pp. 165-69 & 170-74.
Heukelbach, J., A. M. L. Costa, T.
Wilcke & N. Mencke. 2004. The animal reservoir of Tunga penetrans in severely affected communities
of north-east
Brazil. Med. & Vet. Ent. 18: 329-35.
Heukelbach, J., A. Franck & H.
Feldmeier. 2004. High attack rate of Tunga penetrans (L. 1758) infestations in an
impoverished Brazillian community.
Trans. Roy Soc. Trop. Med. & Hyg. 98: 43-44.
Hinkle, N. C., P. G. Koehler, W. H.
Kern & R. S. Patterson. 1991. Hematophagous strategies of the cat flea
(Siphonaptera: Pulicidae).
Florida Ent. 73: 377-85.
Hinkle, N. C., P. G. Koehler & R.
S. Patterson.1995. Residual effectiveness
of insect growth regulators applied to carpet for control of cat flea
(Siphonaptera: Pulicidae) larvae. J. Econ. Ent. 88: 903-6.
Hinkle, N. C., M. K. Rust & D. A. Reierson. 1997.
Biorational approach to flea (Siphonaptera: Pulicidae) suppression:
present and future. J. Agric.
Ent.
14: 309-21.
Hinkle, Nancy C., Philip G. Koehler,
and Richard S. Patterson. 1990. Egg
production, larval development and adult longevity of cat fleas
(Siphonaptera: Pulicidae) exposed to ultrasound. J. Econ. Entomol. 83(6): 2306-2309.
Hubbard, C. A. 1947.
Fleas of western North America.
Ames, Iowa Press.
Lopez-Berrizbeitia, M. F. et al.
2015. A new flea of the genus Ctenidiosomus (Siphonaptera, Pygiopsyllidae)
from Salta Province, Argentina.
Zoo Keys 512: 109-120.
Matheson, R. 1950. Medical Entomology. Comstock Publ. Co, Inc. 610 p.
Pugh, R. E.
1987. Effects on the
development of Dipylidium
caninum and on the host reaction to this parasite in the
adult flea
(Ctenocephalides felis). Parasitol. Res.
73: 171-77.
Rothschild, N. C. 1910.
A synopsis of the fleas found on Mus norvegicus, Mus rattus and Mus musculus.
Bull. Ent. Res. 1: 89-98.
Rust, M. K. 2005.
Advances in the control of Ctenocephalides
felis (cat flea) on cats and dogs. Trends in Parasitol. 1: 232-36.
Rust, M. K. & M. W.
Dryden. 1977. The biology, ecology and management of the
cat flea. Ann. Rev. Ent. 42: 451-73.
Schriefer, M. E., J. B. Sacci, J. P. Taylor, J. A.
Higgins & A. F. Azad. 1994. Murine typhus: updated roles of multiple
urgan components and a second
typhuslike rickettsia. J. Med. Ent. 31: 681-85.
Service, M. 2008.
Medical Entomology For Students.
Cambridge Univ. Press. 289 p
Scott, S. & C. J.
Duncan. 2001. Biology of Plagues: Evidence from Historical Populations. Cmbridge Univ. Press, England.
Traub, R. & H. Starcke (eds.) 1980. Fleas. Proc. Intern. Conf. on Fleas, Ashton Wold,
Peterborough, UK, 21-25 Jun 1977.
Rotterdam: Balkema.
|
