File: <ch-126.htm> GENERAL INDEX [Navigate to MAIN MENU ]
|
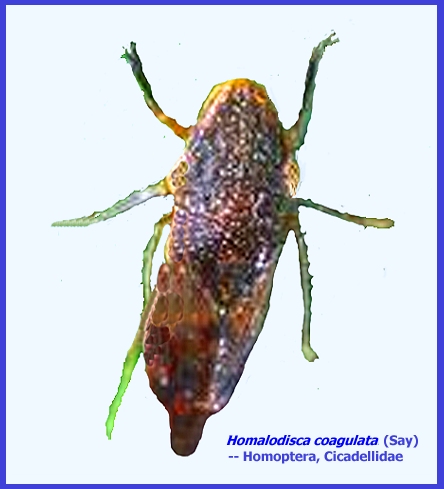
GLASSY WINGED
SHARPSHOOTER Homalodisca coagulata (Say) (Homoptera : Cicadellidae) (Link - University of California) (Contacts) ----- CLICK on Photo to enlarge & search for Subject
Matter with Ctrl/F. GO TO ALL: Bio-Control Cases
The major wine-growing regions of New Zealand, Australia, the Bordeaux region of France, most areas of Spain, as well as central and southern Italy have climates that could be conducive to sharpshooter establishment and proliferation should it be accidentally introduced (Hoddle 2004a). It was likely introduced accidentally on ornamental plants imported from California. Tahiti is the largest and most populous island in the group of islands collectively known as the Windward Islands in the Society Islands Archipelago in French Polynesia in the South Pacific. French Polynesia is very remote; it is 6,000 km west of Chile, and 5,200 km east of Australia. The Cook Islands lie 900 km to the west of French Polynesia and Easter Island is 5,178 km to the east. sharpshooter flourished in Tahiti because of an extremely mild year round climate and an abundant supply of exotic and ornamental plants maintained in gardens. In contrast to California, where there are just two generations [spring and summer] each year, natural enemies are lacking, and no obvious competitors exist in urban or natural settings, sharpshooter populations underwent exponential growth Naturally occurring parasitism of
sharpshooter eggs was very low on the island of Mo’orea, the immediate
neighboring island to Tahiti. Surveys indicated that less than 2% of
individual eggs were attacked by generalist egg parasitoids in just 4% of egg
masses collected. Of those egg masses attacked, 44% of the eggs were
parasitized. Because only a few eggs in an egg mass were attacked (indicating
inefficient and opportunistic exploitation) and only males were reared from
sharpshooter eggs (which suggest poor host quality as females did not
oviposit fertilized female eggs), and the wasp responsible for attacking
sharpshooter egg masses was a species of platygasterid, a family that does
not specialize on sharpshooter eggs, but will parasitize various species of
leafhoppers, indicated there were no native specialized parasitoids attacking
sharpshooter. Consequently, sharpshooter populations in French Polynesia were
free of the pressures associated with natural enemies and this pest reached
extraordinarily high densities in Tahiti. Watery excreta known as
sharpshooter rain, literally rained from infested trees because there were so
many sharpshooter feeding on trees. Such high populations retarded plant
growth and reduced local fruit production. Further, the use of shade trees
was reduced because so much sharpshooter rain made them unsuitable for
escaping the intense tropical sun. Finally, at night, incredible numbers of
sharpshooter would be attracted to lights and large numbers would fly into
homes, shops, and business at night. This was very annoying because adult
sharpshooter would die and need to be swept up, the wingbeat frequency of
adults flying past heads caused an unpleasant “buzz” in ears, and adults
would occasionally “bite” people when they landed on exposed skin and probed
with their needle-like mouthparts. Because of high levels of human activity and interisland movement of plants between Tahiti and other islands in French Polynesia, sharpshooter was moved rapidly to other island groups in French Polynesia. In 2001, it was found in Raiatea (Leeward Islands) and in 2002 in Moorea. Populations were then discovered in the Leeward Islands of Huahine and Bora Bora in 2003, and in Tahaa and Maupiti in 2005. At the end of 2004 and the beginning of 2005, sharpshooter populations were discovered outside of the Society Islands in two other archipelagos of French Polynesia substantially distant from Tahiti: the Australs, where two islands were infested (Rurutu and Tubuai) (January 2005) and the Marquesas, where one island, Nuku Hiva, was found infested in November 2004. To combat the sharpshooter
infestation in French Polynesia the best control to use would be classical
biological control. To this end, the mymarid egg parasitoid Gonatocerus
ashmeadi was imported into quarantine in Tahiti in September
2004 and studied to make sure it did not pose any undue risk to other insects
already present in French Polynesia. Following these studies, the accumulated
evidence strongly indicated that the parasitoid was likely safe for release
and posed no undue risk to non-target species in French Polynesia. The
parasitoid was released on May 2, 2005 on Tahiti. Between May and October 2005, 13,786 parasitoids were released
at 27 sites in Tahiti. The parasitoid established readily, and within seven
months of release sharpshooter had been completely controlled in Tahiti and
sharpshooter populations were reduced by over 95%. The parasitoid also spread
unassisted to every other island infested by sharpshooter. This rapid
movement between islands strongly suggested that quarantines that were
established to reduce sharpshooter were not working and people were still
moving plants infested with sharpshooter eggs. However, this time eggs were
parasitized by G. ashmeadi, and this is
how it was moved unintentionally between islands and archipelagos. Reduction of sharpshooter populations on Tahiti following the release of G. ashmeadi. (A) sharpshooter densities on Tahtiti from one minute sweep net sampling of hisbiscus bushes before the release of G. ashmeadi on May 2 2005; (B) sharpshooter densities five months (October 1 2005) after parasitoid release; (C) sharpshooter densities seven months after parasitoid release (December 12 2005); (D) sharpshooter densities two years after the release of G. ashmeadi (Figure courtesy of J. Grandgirard and J. Petit.). [Further Details.] REFERENCES Barratt, B.I.P., Howarth, F.G.,
Withers, T.M., Kean, J.M., Ridley, G.S., 2010. Progress in risk assessment
for classical biological control. Biological Control 52: 245–254. Bartlett, B. R. 1962. The ingest ion
of dry sugars by adult entomophagous insects and the use of this feeding
habit for measuring the moisture needs of parasites. Journal of Economic
Entomology 55 (5): 749-753. Berndt, Lisa A., Wratten, Steve D. 2005. Effects of alyssum flowers on the longevity, fecundity,
and sex ratio of the leafroller parasitoid Dolichogenidea tasmanica.
Biological Control 32 (1): 65-69. Boyd, E. A., Hoddle, M.S. 2007. Host
specificity testing of Gonatocerus spp.
egg-parasitoids used in a classical biological control program against Homalodisca
vitripennis: A retrospective analysis for non-target impacts
in southern California. Biological Control 43: 56-70. Carbone, S. S., Rivera, A. C. 2003.
Egg load and adaptive superparsitism in Anaphes nitens,
an egg parasitoid of the Eucalyptus snout-beetle Gonipterus scutellatus.
Entomologia
Experimentalis et Applicata 106: 127–134. CDFA. 2003. Pierce's disease control
program - report to the legislature, May 2003 [Online]. Available at:
http://www.cdfa.ca.gov/phpps/pdcp/docs/2002LegReport.pdf. (verified 8 July
2004) CDFA, 2008. Pierce’s Disease Program
Annual Report to the Legislature 2008. California Department of Food and
Agriculture, Sacramento, CA, USA. Chaney, W.E. 1998. Biological control
of aphids in lettuce crops using in-Weld insectaries. In: Pickett, C.H.,
Bugg, R.L. (Eds.), Enhancing Biological Control—Habitat Management to Promote
Natural Enemies of Agricultural Pests. University of California Press,
Berkeley, CA, pp. 73–83. Chen, Wen-Long, Leopold, Roger A.,
Morgan, David J. W., et al. 2006a. Development and reproduction of the egg
parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera :
Mymaridae), as a function of temperature. Environmental Entomology 35 (5):
1178-1187. Chen, W. L., Leopold, R. A., Harris,
M. O. 2006b. Parasitism of the glassy-winged sharpshooter,
Homalodisca coagulata (Homoptera : Cicadellidae): Functional
response and superparasitism by Gonatocerus ashmeadi
(Hymenoptera : Mymaridae). Biological Control 37 (1): 119-129. Cheou D. 2002. Incursion of
glassy-winged sharpshooter Homalodisca coagulata in
French Polynesia. Plant Protection Service Pest Alert:1. Costa H.S., Blua, M.J., Bethke, J.A.,
Redak, R.A. 2000. Transmission of Xylella fasitidiosa to
oleander by the glassy-winged sharpshooter, Homalodisca coagulata.
HortScience 35(7):1265-1267. De León, J.H., Logarzo, G.A., and
Triapitsyn, S.V. (2008), Molecular characterization of Gonatocerus
tuberculifemur (Ogloblin) (Hymenoptera: Mymaridae), a
prospective Homalodisca vitripennis (Germar) (Hemiptera:
Cicadellidae) biological control candidate agent from South America:
divergent clades. Bulletin of Entomological Research 98: 97-108. de Lima, J. E. O., Miranda, V. S.,
Hartung, J. S., Brlansky, R. H., Coutinho, A., Roberto, S. R., Carlos, E. F.,
1998. Coffee
leaf scorch bacterium: Axenic culture, pathogenicity, and comparison with Xylella
fastidosa of citrus. Plant Disease 82: 94-97. Eilenburg, J., Hajek, A., Lomer, C.,
2001. Suggestions for unifying the terminology in biological control.
Biocontrol 46: 387-400. Ellers, Jacintha, Van Alphen, Jacques
J. M., Sevenster, Jan G. 1998. A field study of size-fitness relationships in
the parasitoid Asobara tabida. Journal of
Animal Ecology 67 (2): 318-324. Esser, T. West, D. (eds.), 2010.
Pierce’s Disease Control Symposium Proceedings, December 15-17, San Diego,
California, pp. 283. California Department of Food and Agriculture,
Sacramento, CA. Available from:
http://www.cdfa.ca.gov/pdcp/2010_Research_Proceedings.html. Flanders, Stanley E. 1965. On the sexuality and sex ratios of hymenopterous
populations. American Naturalist 99 (909): 489-494. Gurr, G. M., Scarratt, S. L., Wratten,
S. D., Berndt, L., Irvin, N. 2004. Ecological engineering, habitat manipulation
and pest management. In: Gurr, G. M., Wratten, S. D., Altieri, M. A. (Eds.),
Ecological Engineering for Pest Management: Advances in Habitat Manipulation
for Arthropods, CSIRO Publishing, Collingwood, VIC, Australia, pp. 1-12. Gurr G.M., Wratten, S.D., Luna, J.M.
2003. Multi-function agricultural biodiversity: Pest management and other
benefits. Basic & Applied Ecology 4(2):107-16. Hartung, J. S., Beretta, J., Brlansky,
R. H., Spisso, J., Lee, R. F., 1994. Citrus variegated chlorosis bacterium:
Axenic culture, pathogenicity, and serological relationships with other
strains of Xylella fastidiosa. Phytopathology 84: 591-597. Hernandez-Martinez, Rufina, de la
Cerda, Karla A., Costa, Heather S., et al. 2007. Phylogenetic relationships of Xylella fastidiosa
strains isolated from landscape ornamentals in southern California.
Phytopathology 97 (7): 857-864. Hoddle M.S. 2004b. Restoring balance:
Using exotic natural enemies to control invasive exotic species. Conservation
Biology 18 (1): 38-49. Hoddle, M. S., Triapitsyn, S. V., 2004. Searching for and collecting
egg parasitoids of the glassy-winged sharpshooter in the central and eastern
USA. In: Tariq, M. A., Oswalt, S., Blincoe, P., Ba, A., Lorick, T., Esser, T.
(Eds.), Proceedings of the Pierce’s Disease Research Symposium. California
Department of Food and Agriculture, 7-10 December 200, San Diego, CA., pp.
342-344. Hokkanen, H.M.T., and Pimentel, D. 1989. New associations in biological control: theory and practice. Canadian Entomologist 121: 829-840. Hoover W. 2004. New invader may
threaten crops. The Honolulu Advertiser, May 14, 2004, Honolulu. Huber, J. T. 1988. The species groups of Gonatocerus Nees
in North America with a revision of the Sulphuripes and Ater groups
(Hymenoptera: Mymaridae). Memoirs of the Entomological Society of Canada 141:
109. Irvin, Nicola A., Hoddle, Mark S.
2004. Oviposition preference of Homalodisca coagulata for
two Citrus limon cultivars and influence of host plant on parasitism by Gonatocerus
ashmeadi and G. triguttatus (Hymenoptera:Mymaridae).
Florida Entomologist 87 (4): 504-510. Irvin N., Hoddle, M.S. 2005a.
Determination of Homalodisca coagulata
(Hemiptera: Cicadellidae) egg ages that are suitable for oviposition by Gonatocerus
ashmeadi, G. triguttatus and G.
fasciatus (Hymenoptera: Mymaridae): (1) no choice tests.
Biological Control 32 (3): 391-400. Irvin, N. A., Hoddle, M. S. 2005b. The
competitive ability of three mymarid egg parasitoids (Gonatocerus
spp.) for glassy-winged sharptshooter (Homalodisica
coagulata) eggs. Biological Control 34 (2): 204-214. Irvin, N. A., Hoddle, M. S. 2006. The
effect of intraspecific competition on progeny sex ratio in Gonatocerus
spp. for Homalodisca coagulata egg
masses: Economic implications for mass rearing and biological control.
Biological Control 39 (2): 169-170. Irvin, Nicola A., Hoddle, Mark S.
2007. Evaluation of floral resources for enhancement of fitness of
Gonatocerus ashmeadi, an egg parasitoid of the glassy-winged
sharpshooter, Homalodisca vitripennis.
Biological Control 40 (1): 80-88. Irvin, Nicola A., Hoddle, Mark S.
2009. Egg maturation, oosorption, and wing wear in Gonatocerus ashmeadi
(Hymenoptera: Mymaridae), an egg parasitoid of the glassy-winged
sharpshooter, Homalodisca vitripennis
(Hemiptera: Cicadellidae). Biological Control 48 (2): 125-132. Irvin, N. A., Hoddle, M. S. 2010.
Comparative assessments of Gonatocerus ashmeadi and
the 'new association' parasitoid Gonatocerus tuberculifemur
(Hymenoptera: Mymaridae) as biological control agents of Homalodisca
vitripennis (Hemiptera: Cicadellidae). Biological Control 55
(3): 186-196. Irvin, Nicola A., Hoddle, Mark S.,
Castle, Steven J. 2007. The effect of resource provisioning and sugar
composition of foods on longevity of three Gonatocerus spp.,
egg parasitoids of Homalodisca vitripennis.
Biological Control 40 (1): 69-79. Irvin, N. A., Hoddle, M. S., Morgan,
D. J. W. 2006. Competition between Gonatocerus ashmeadi and G.
triguttatus for glassy-winged sharpshooter (Homalodisca
coagulata) egg masses. Biocontrol Science and Technology 16
(4): 359-375. Irvin, N.A., Hoddle, M.S.,
Suarez-Espinoza, J., 2009. The functional response of Gonatocerus
ashmeadi and the ‘new association’ parasitoid G.
tuberculifemur attacking eggs of Homalodisca vitripennis.
Environmental Entomology 38 (6): 1634–1641. Jervis, Mark A., Heimpel, George E., Ferns, Peter N., et al. 2001. Life-history strategies in parasitoid wasps: A comparative analysis of 'ovigeny'. Journal of Animal Ecology 70 (3): 442-458. Johnson, J. B., Stafford, M. P. 1985.
Adult Noctuidae feeding on aphid honeydew and a discussion of honeydew
feeding by adult Lepidoptera. Journal of the Lepidopterists' Society 39 (4):
321-327. Jones, W. A., Logarzo, G. A.,
Triapitsyn, S.V., Casas, M., Virla, E. G., Purcell, A.H., 2005a. Biology and host range of two South American egg
parasitoids (Hymenoptera: Mymaridae), possible biocontrol agents for
glassy-winged sharpshooter (Say) (Hemiptera: Cicadellidae: Proconiini).
Proceedings of the 12th International Auchenorrhyncha Congress and 6th
International Workshop on Leafhoppers and Planthoppers of Economic
Significance, University of California, Berkeley, 7-12 August 2005, Berkeley,
CA, Available online at http://nature.berkeley.edu/hoppercongress/ Jones, W. A., Logarzo, G. A.,
Virla, E. G., Luft, E., 2005b. Environmental risk assessment of egg parasitoids from South America:
non-target field and laboratory host range in Argentina and the U. S. In:
Tariq, M. A., Blincoe, P., Mochel, M., Oswalt, S., Esser, T. (Eds.), Proceedings
of the Pierce’s Disease Research Symposium. California Department of Food and
Agriculture, 5-7 December 2005, San Diego, CA, pp. 343-344. Kazmer, David J., Luck, Robert F.
1995. Field tests of the size-fitness hypothesis in the egg parasitoid Trichogramma
pretiosum. Ecology 76 (2): 412-425. Krugner, R., Johnson, M. W., Groves,
R. L., Morse, J. G. 2008. Host specificity of Anagrus epos:
a potential biological control agent of Homalodisca vitripennis.
Biocontrol 56: 439-449. Krugner, R., Johnseon, M. W., Morgan,
D. J. W., Morse, J. G. 2009. Production of Anagrus epos
Girault (Hymenoptera: Mymaridae) on Homalodisca vitripennis
(Germar) (Hemiptera: Cicadellidae) eggs. Biological Control 51: 122-129. Landis D.A., Wratten, S.D., Gurr, G.M.
2000. Habitat management to conserve natural enemies of arthropod pests in
agriculture. Annual Review of Entomology 45:175-201. Messenger, P.S., and van der Bosch, R.
(1971), The adaptability of introduced biological control agents. In: Huffaker,
C.F. (ed.), Biological Control, Plenum Press: New York, pp. 68-92. Miller, W. E. 1989. Reproductive enhancement by adult feeding effects of
honeydew in imbibed water on spruce budworm. Journal of the Lepidopterists'
Society 43 (3): 167-177. Montero-Astua, M., Saborio, G. R.,
Chacon-Diaz, C., Garita, L., Villalobos, W., Hartung, J. S., Rivera, C.,
2008. First
report of Xylela fastidosa in Avocado in Costa Rica. Plant Disease 92 (1):
175. Pilkington, L.J., Irvin, N.A., Boyd,
E.A., Hoddle, M.S., Triapitsyn, S.V., Carey, B.G., Jones, W.A., Morgan,
D.J.W., 2005. Introduced parasitic wasps could control glassy-winged
sharpshooter. California Agriculture 59 (4): 223–228. Pilkington, Leigh J., Hoddle, Mark S.
2006. Reproductive and developmental biology of Gonatocerus ashmeadi
(Hymenoptera : Mymaridae), an egg parasitoid of Homalodisca coagulata
(Hemiptera : Cicadellidae). Biological Control 37 (3): 266-275. Pimentel, D. 1963. Introducing
parasites and predators to control native pests. Canadian Entomologist 95:
785-792. Riddick, E. W. 2005. Are lab-cultured Anaphes
iole females strictly proovigenic? BioControl 50 (6): 911-919. Salt, G., 1961. Competition among insect parasitoids: mechanisms in
biological competition. Symposium of the Society for Experimental Biology 15:
96-119. Siebert J., 2001. Economic impact of
Pierce's disease on the California grape industry. Pierce's Disease Research
Symposium: 111-116. Simberloff, D., Stiling, P., 1996. How
risky is biological control? Ecology 77 (7): 1965–1974. Sorensen J.T., Gill, R.J. 1996. A
range extension of Homalodisca coagulata
(Say) (Hemiptera: Clypeorrhyncha: Cicadellidae) to southern California.
Pan-Pacific Entomologist 72 (3):160-1. Triapitysn, S. V. 2006. A key to the
Mymaridae (Hymenoptera) egg parasitoids of proconiine sharpshooters
(Hemiptera: Cicadellidae) in the Nearctic region, with description of two new
species of Gonatocerus. Zootaxa 1202:1-38. Triapitsyn, S.V., Logarzo, G.A., De
León, J.H., Virla, E.G., 2008. A new Gonatocerus (Hymenoptera: Mymaridae) from Argentina,
with taxonomic notes and molecular data on the G. tuberculifemur
species complex. Zootaxa 1949: 1–29. Triapitsyn S.V., Morgan, D.J.W.,
Hoddle, M.S., Berezovskiy, V.V. 2003. Observations on the biology of Gonatocerus
fasciatus Girault (Hymenoptera : Mymaridae), egg parasitoid
of Homalodisca coagulata (Say) and Oncometopia
orbona (Fabricius) (Hemiptera : Clypeorrhyncha :
Cicadellidae). Pan-Pacific Entomologist 79 (1):75-76. Triapitsyn S.V., Phillips, P.A. 2000.
First record of Gonatocerus triguttatus
(Hymenoptera : Mymaridae) from eggs of Homalodisca coagulata
(Homoptera : Cicadellidae) with notes on the distribution of the host.
Florida Entomologist 83 (2): 200-203. Velema, H.P., Hemerik, L., Hoddle,
M.S., and Luck, R.F. (2005). Brochosome influence on parasitisation
efficiency of Homalodisca coagulata
(Say) (Hemiptera : Cicadellidae) egg masses by Gonatocerus ashmeadi Girault
(Hymenoptera : Mymaridae). Ecological Entomology 30: 485-496. Vickerman D.B., Hoddle, M.S., Triapitysn, S.V., Stouthamer, R. 2004.
Species identity of geographically distinct populations of the glassy-winged
sharpshooter parasitoid Gonatocerus ashmeadi:
Morphology, DNA sequences and reproductive compatibility. Biological Control
31 (3): 338-345. Zwolfer, H., 1971. The structure and effect of parasite complexes attacking phytophagous host insects. In: denBoer, P. J., Gradwell, G. R. (Eds.), Dynamics of Populations: Proceedings of the Advanced Study Institute on Dynamics and Numbers in Populations. Oosterbeck 1970. Centre for Agricultural Publicating and Documentation, Wageningen, The Netherlands, pp. 405-418. |