File: <bc-51b.htm> Pooled References GENERAL
INDEX [Navigate
to MAIN MENU ]
MORPHOLOGY OF INSECTS
(With
Emphasis on Natural Enemy Identification)
(Contacts)
----Please
CLICK on desired
underlined categories to view, or on images to enlarge
[To search
for
Subject
Matter, depress Ctrl/F ]
Summary
Insect Morphology is presented for the
purpose of instructing those interested in the identification of insects,
particularly species with predatory or parasitic behavior. The evolutionary format used is to ease
the means by which the various insect structures may be learned. The text is produced or
paraphrased from cited references. It was developed by the author while at
the University of Wisconsin and Utah State University, The diagrams were derived and modified from
those provided of the author and Dr. Robert Dicke at the University of
Wisconsin, Madison and Dr. Donald Davis, Utah State University. The
terminology of Snodgrass (1952) is generally used. Acknowledgment and appreciation
are made to the following who assisted during the course work and later
developmental phases: Dr. D. P. Annecke, Dr. Blair R. Bartlett, Dr. Robert F. Brooks,
Dr. Donald W. Clancy, Dr. Curtis P. Clausen, Dr. Harold Compere, Dr. John
Falter, Dr. Stanley E. Flanders, Dr. C. A. Fleschner, Dr. Dan Gerling, Dr.
Gordon Gordh, Dr. Marcos Kogan, Dr. Clayton W. McCoy, Dr. David Rosen &
Dr. G. Zinna. Special appreciation is
extended to Dr. Dorothy Feir who supplied some of the early drawings of Dr.
Dicke, which had become lost. - - - - - - - - - - - - - - - - - - - - - -
- - - - - - Introduction
Insect identification to the
specific level requires a substantial knowledge of morphology. The following is an introduction to the
gross, comparative morphology of insects.
The term, morphology as developed in this work is a study of the
functional form of an insect, although details of anatomy or the specific
parts of an insect must be described before the functional whole can be
grasped. It is a comparative
morphology restricted to seven representative species that were chosen to
broadly represent the complex spectrum of insect forms. These are in ascending evolutionary
sophistication, Silverfish, Thermobia
domestica (Packard) ‑ Thysanura; Madeira roach, Leucophaea maderae (Fabricius) ‑
Orthoptera; Milkweed bug, Oncopeltus
fasciatus (Dallas) ‑ Hemiptera; June beetle, Phyllophaga rugosa (Melsheimer) ‑ Coleoptera; Noctuid moth,
Heliothis zea (Boddie) ‑
Lepidoptera; House fly, Musca domestica
(Linnaeus) ‑ Diptera; and the Honey bee, Apis mellifera (Linnaeus) ‑ Hymenoptera The general plan of this study
establishes a typical insect form for comparative purposes, which basically
represents most insects, as we know them today. The cockroach, Leucophaea
maderae, was arbitrarily selected by Dr. Robert Dicke as such a
"typical" form. This selection was based on concepts of the
evolutionary changes that probably occurred from a hypothetical worm‑like
ancestor through the primitive silverfish, to the very highly evolved or specialized
house fly and honey bee. A primitive structure or
system is one that has occurred early in the evolutionary history of insects,
while a specialized structure is a more
recent elaboration of a primitive form.
The establishment of a concept of A primitive structure facilitates
comparisons or homologies and allows an understanding of specializations that
have given insects as a group such a wide range of successful adaptation to
their environment. However, the
concept or designation of primitive does not imply relative uselessness. A vestige is
a useless relic of postevolutionary development. Although a primitive structure may have occurred early in
evolutionary history as a very useful, it may be retained by an otherwise
highly evolved form. The giant
tropical cockroach, Leucophaea maderae,
representing a group of Orthoptera which probably evolved very early in
insect history will serve as the typical form. Thermobia domestica
represents a group of primitively wingless Thysanura illustrates many of the
theoretical primitive structures. The
milkweed bug, Oncopeltus fasciatus
is an insect that has retained the primitive wing development and
metamorphosis of Leucophaea maderae,
but also shows considerable evolutionary change in the structure of the head
and mouthparts. Phyllophaga rugosa, Heliothis
zea, Musca domestica and Apis mellifera are representatives of
the four major orders of insects.
These illustrate many specializations, especially in the metamorphic
forms or larvae that precede the adult stage. The detailed drawings in the text
are useful during dissections and study of preserved and living insects in
the manner that an artisan would employ a set of blueprints in his
construction of a building or machine.
The descriptive text should be studied, the structures identified, and
the concepts verified by examination of the drawings. However, all this effort is incomplete at
best until one has personally dissected, manipulated and identified the
animal's structures and systems. Theoretical
concepts are alluded to and then thoroughly discussed in Section IV. All technical terms are in bold faced type
and specifically described in Section VII,
Morphological Terminology. Dr. Robert Dicke in his course
"Insect Morphology" at the University of Wisconsin, concluded with
the following introductory comments, "Proceed carefully and diligently
with your study and dissection of these insects. You will be rewarded by a fascinating display of an ingenious
and beautifully created machinery that can sense and adapt itself to a
complex environment, that can ingest and synthesize a wide range of organic
matter, and that comprises a vast group of animals which probably will
reproduce and survive in spite of the intentional or incidental efforts of
man to exterminate them." EXTERNAL MORPHOLOGY
SECTION I ‑ THE BODY WALL
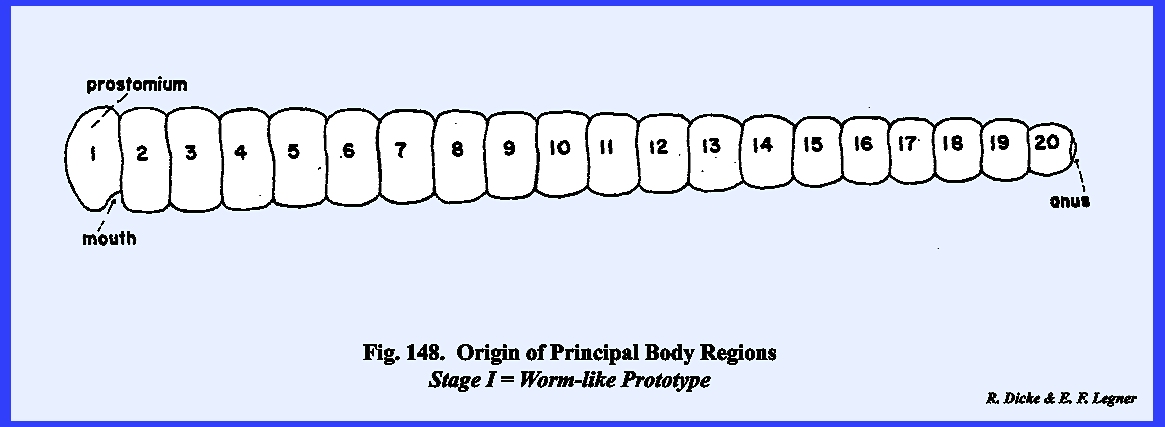
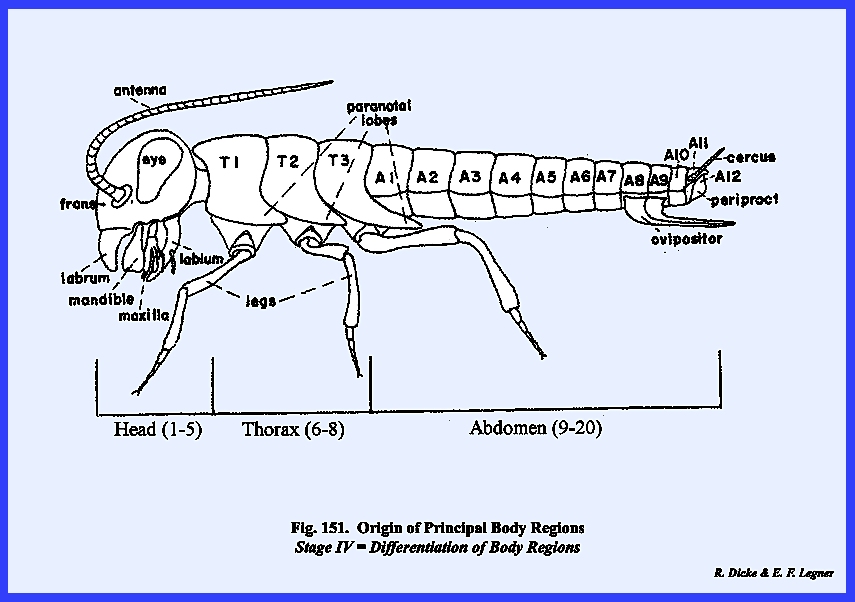
Metamerism and the Principal Body Regions A major characteristic of an
Arthropod is the division of its body into segments. This trunk segmentation is usually
referred to as metamerism. Each body segment may then be identified as a metamere. Considerable evidence exists that all
Arthropods including insects probably evolved from a segmented, worm‑like
ancestor or prototype comprising about 20 distinct
but undifferentiated metameres./1
Each metamere probably was cylindrical or ring‑like in form, and
in a series coextensive with the gut or intestinal tract was joined together
by transverse invaginations of the body wall. The anterior opening to the gut or mouth was
probably situated ventrally between the first metamere or prostomium and
second metamere, while the posterior opening to the gut or anus was
borne by the last metamere or periproct. With the exception of the periproct, each metamere acquired a
pair of ambulatory appendages by means of
lateral expansions of the body wall.
It is then believed that this prototype evolved into the present day
insect form through a series of specializations in which distinct functions
of the organism became the responsibility of certain body regions. These body regions or tagmata
are the head (region of ingestion and principal sensory perception), the
thorax (region of locomotion), and the abdomen
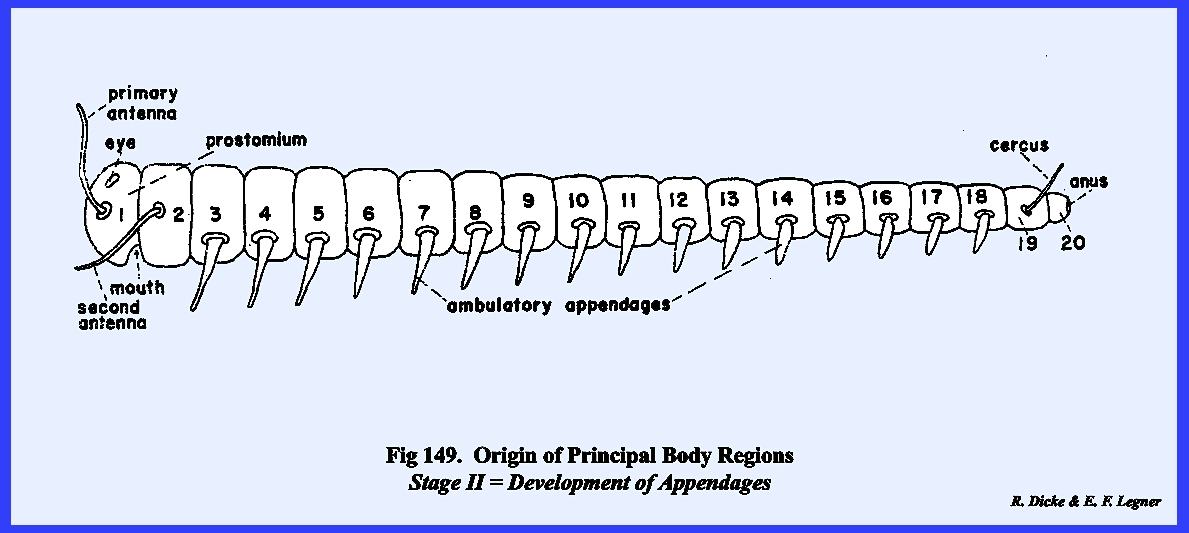
(region of visceral function and reproduction). The prostomium and first four metameres are thought to have
coalesced into the head region. The
locomotory appendages of the prostomium probably evolved into sensory
structures or antennae and the three appendages of the
posterior metameres of the head complex became modified into organs of
ingestion or, the mouthparts. Fusion of
the metameres of the head region has been so complete that no evidence of
their separate entities exists in present day forms. The 6th, 7th and 8th metameres comprise
the thoracic region. In most insect
forms, lateral appendages of this region were retained and further
specialized to become the principal organs of locomotion. Wings, as additional expansions of the
body wall, provided highly specialized and unique forms of locomotory
structures. Complex external and
internal modifications of the thoracic metameres were required to support and
propel the leg and wing mechanisms.
The remaining metameres of the hypothetical prototype were evolved
into the abdominal tagma. With few
exceptions, the ambulatory functions of the lateral appendages of the
abdominal metameres were lost or modified into specialized appendages,
especially for the reproductive function.
The abdominal region, devoted primarily to housing the principal
visceral systems, retained many of the features of the undifferentiated
primitive metamere. A preliminary
examination of the body form of the representative insect species included
here will demonstrate that the three body tagmata are distinct even in the
caterpillar of Heliothis zea. However, extreme modifications are quite
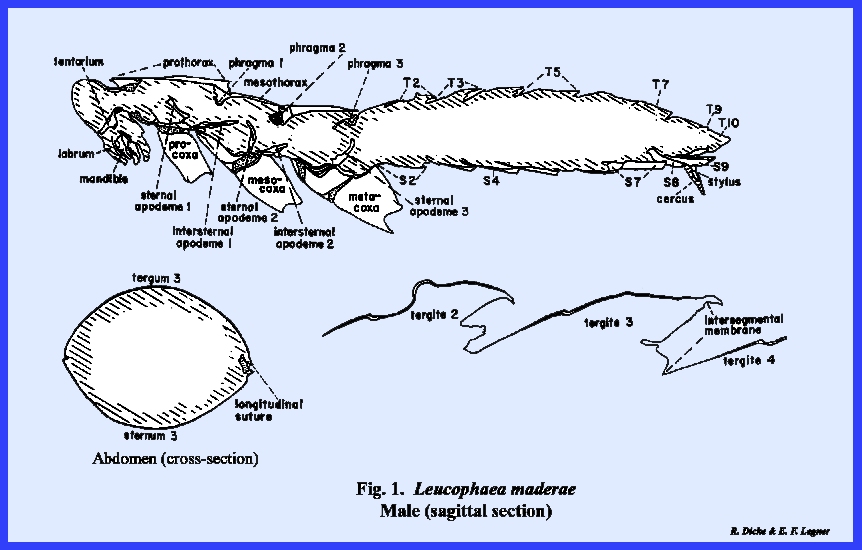
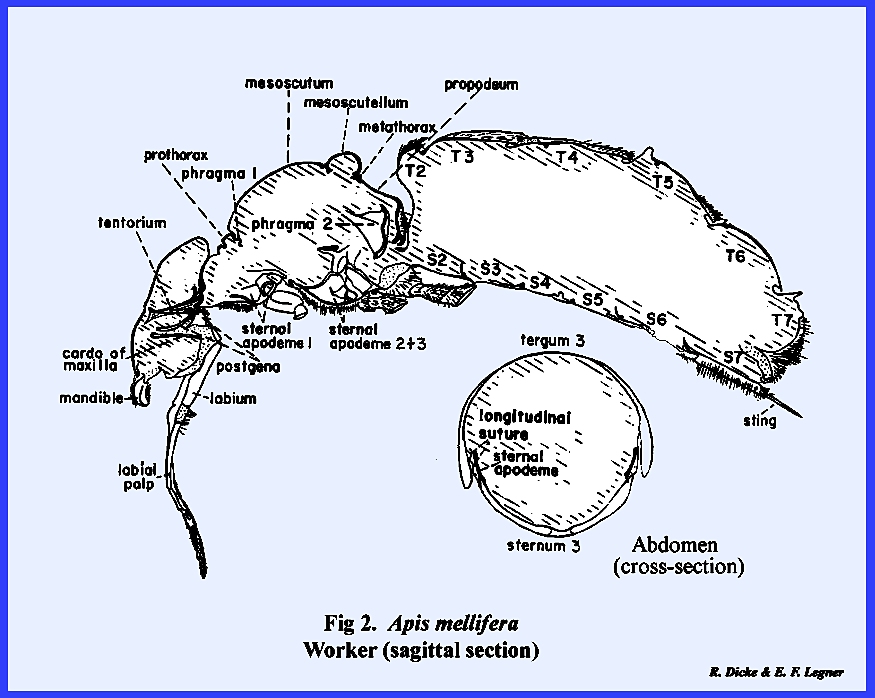
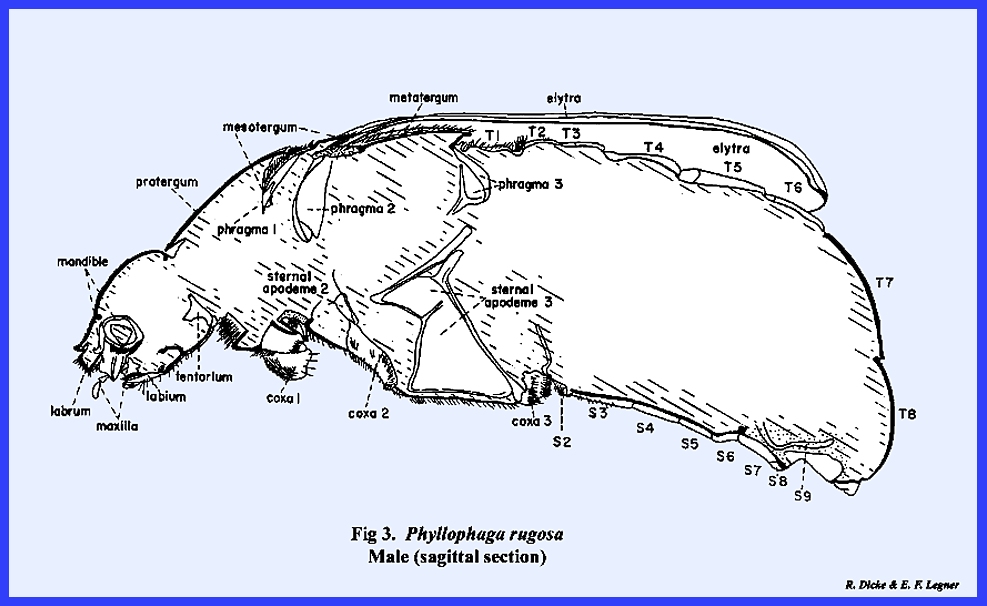
apparent in the illustrated sagittal sections of Leucophaea maderae (Fig 1), Apis mellifera (Fig
2) and Phyllophaga rugosa (Fig
3). The body of Leucophaea maderae is flattened, or dorso‑ventrally
compressed, and an outline of the thoracic and at least the first eight
abdominal metameres are comparable in size and form. In contrast, the abdomen of Apis mellifera is cylindrical, and the
number of abdominal metameres is reduced.
An extreme modification of the first abdominal metamere has occurred
(fusion with the thorax, e.g., propodeum, and narrow
petiolated constriction). A
disproportionate development of the 2nd thoracic metamere has evolved along
with wing development at the expense of the first and 3rd (prothorax and
metathorax). The
Exoskeleton
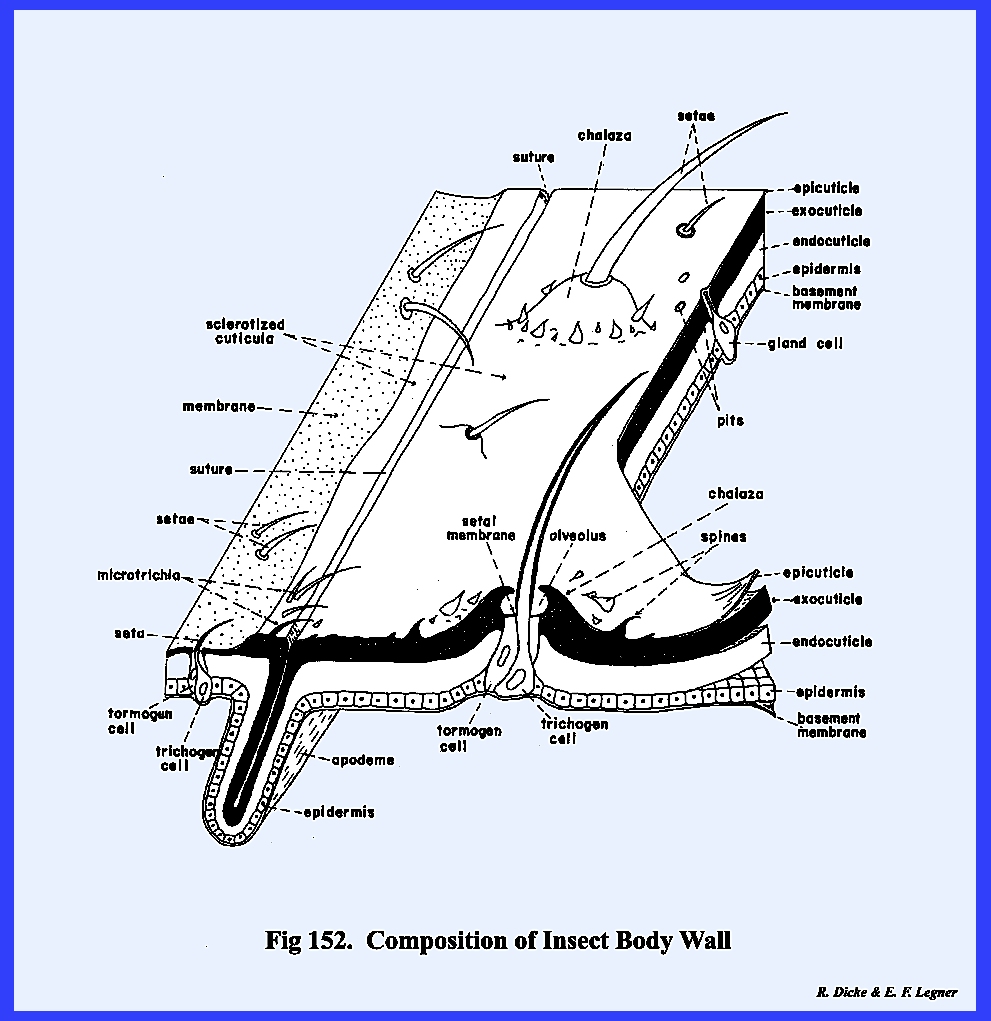
The body wall or integument is the
external covering of an organism which maintains its characteristic form and
contains the body fluids and tissue systems (Fig. 152): In an insect, the integument
further serves the purpose of support as a skeletal system and is an integral
part in the mechanism of locomotion.
The inner cellular layer or epidermis
of the integument secrets an external layer or cuticula./2 This cuticula is composed principally of
a complex of polymerized proteins, a nitrogenous polysaccharide commonly
referred to as chltin, pigments and lipids. The entire external surface of the insect
(as well as such invaginations of the body wall as the fore and hind gut and
genital pouch) is covered by a layer of cuticula. This continuous envelope of cuticula, which incases the insect,
is part of the integument, which is caste and replaced when the body size is
increased by growth. Cuticula may be
soft and flexible or hard and rigid.
The degree of hardening and inflexibility is known as sclerotization.
A sagittal section of an insect's body demonstrates that the
integument serves as its skeletal structure.
Compared with the internal bony skeleton of a vertebrate, this structural
mechanism is the exoskeleton. Thickness of cuticula and the degree of
hardening or sclerotization varies considerably. In Phyllophaga rugosa,
the cuticula of the head and protergum is much thicker than similar areas in Leucophaea maderae. The skeletal structure of a
metamere is not a simple inflexible ring of cuticula. Although the abdominal metameres are the
least modified from the hypothetical form, at least two divisions of the
metamere are apparent as shown in the cross sectional illustrations of Leucophaea maderae (Fig 1) and Apis mellifera (Fig 2). A dorsal plate or tergum is
separated by a longitudinal infolding of the body wall from a ventral plate or sternum. This comparatively thin and flexible
infolding of the body wall is termed a suture. Each of these plates or other areas of the
body wall defined or separated by a suture are collectively termed
sclerites. The metameres of the
thoracic region are further subdivided into sclerites to make up the complex
ambulatory and flight mechanism. A
thoracic metamere is almost box‑shaped, and besides a tergum and
sternum there is a side area or pleura.
The tergum, sternum and pleura are rarely simple plates, but are
further subdivided into sclerites especially on the wing bearing metameres. The
Endoskeleton
The cuticula is more than an outer
skin or protective armor. The body
wall may be invaginated to form cuticular ridges or rods wherever additional
rigidity of the skeletal structure is advantageous Pooled Referencessclerotized. They are called apodemes
and collectively comprise the endoskeleton. Apodemes may be simple internal ridges
such as the dorsal invaginations between the thoracic metameres of Leucophaea maderae (Fig 1). These dorsal thoracic invaginations may
be greatly expanded into a broad plate‑like structure or phragma for muscle attachment as illustrated for Apis mellifera (Fig
2) or Phyllophaga rugosa (Fig 3). Rod‑shaped apodemes may combine to
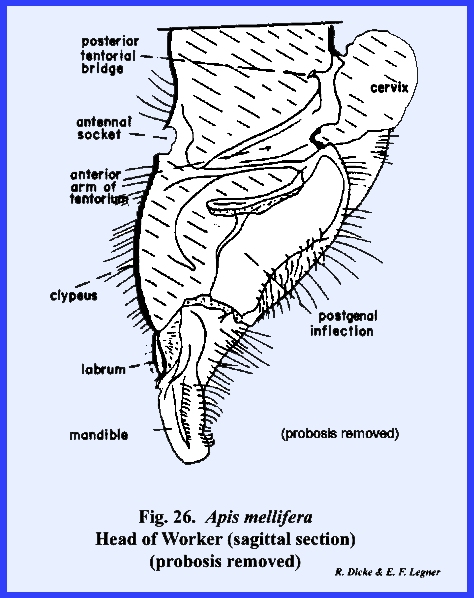
form an effective brace or strut bridging the anterior head cavity. This structure is the tentorium situated
at the base of the mouthparts in Leucophaea
maderae (Fig
1).
Sternal apodemes may be rod‑shaped or forked such as the sternal
and intersternal apodemes of Leucophaea
maderae (Fig
1), or they may be a greatly expanded
median plate such as the sternal apodeme #3 of Phyllophaga rugosa (Fig 3), or
sternal apodeme #2 + 3 of Apis
mellifera. If the apodeme is an
internal ridge or a phragma, the external evidence of such an invagination is
an impression of the body wall. If this
is a shallow groove or impressed line, it may be properly referred to as a
suture. However, if the site of this
invagination is a deep furrow, it is usually referred to as a sulcus. Where the apodeme is a rod or
tubular structure, its point of invagination may be called a pit, e.g., tentorial pits of the head tagma. Not all of the cuticular invaginations are
sclerotized. Soft, flexible
invaginations or intersegmental membranes occur
between the metameres. These
membranes may be pleated and folded as illustrated for the abdominal
metameres of Leucophaea maderae (Fig 1). The intersegmental membranes permit
articulation of the metameres and expansion of the abdominal cavity. This abdominal expansion in insects is rarely
accomplished by a stretching of the body wall. Cuticula when stretched does not fully regain its original
form. Expansion of the abdomen is
accomplished by an unfolding of the intersegmental membranes. A longitudinal suture accomplishes
articulation or expansion between the tergal and sternal sclerites of the
abdomen. Protuberances
of the Body Wall
The external surface of the
cuticula is rarely smooth. In
addition to the more apparent protuberances, the cuticula may be variously
sculptured with minute depressions, corrugations and striations, or by
irregularly alternating concave and convex surfaces. The cuticula may be produced into heavily
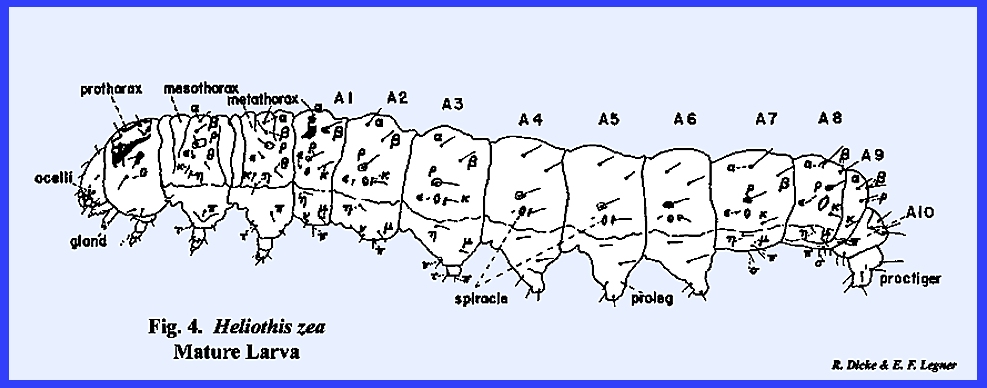
sclerotized spines such as in the caterpillar of Heliothis zea (Figs 4
&
5): The spines may be sharply pointed
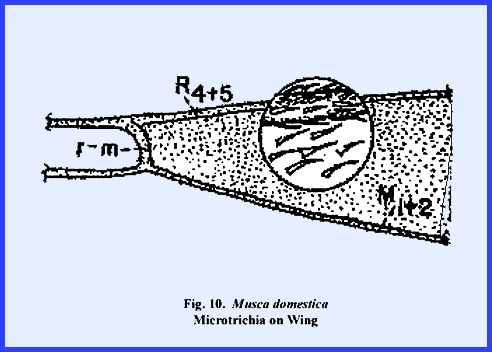
or they may be blunt and irregularly shaped knobs. Spines often resemble minute hairs and are referred to as microtrichia (Fig 6). The veins and wing membrane of Musca domestica have a scattered
covering of microtrichia (Fig
10). Although
spines usually occur in an irregular pattern, they may be arranged in
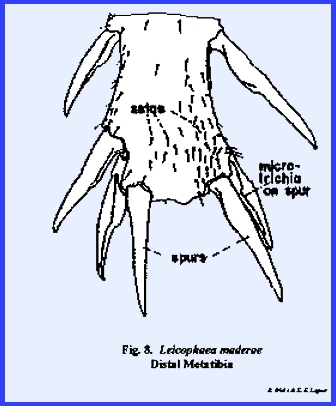
well-defined lines such as on the tibial spurs of Leucophaea maderae (Fig 8). or on the ental surface of the
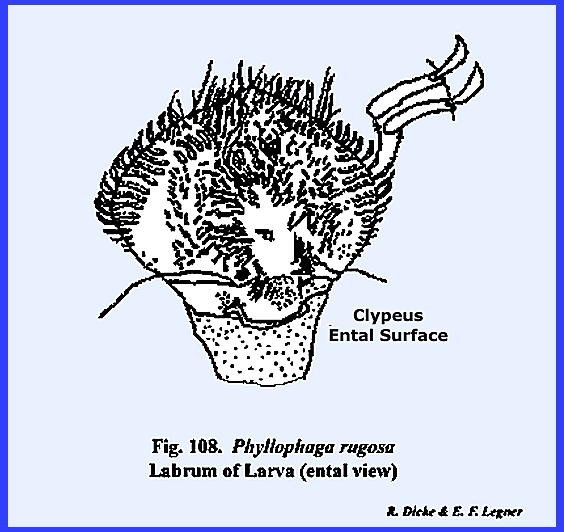
labrum in the grub of Phyllophaga
rugosa (Fig 108): All of these structures are
collectively referred to as noncellular processes since the
protuberance is composed entirely of heavily sclerotized cuticula and are
fixed to and confluent with the exoskeleton. Frequently, the epidermal cells of
the body wall may become modified for the specialized function of secreting
single hollow protuberances or unicellular
processes. These may exhibit a
variety of forms and are referred to by many descriptive terms. The hair like movable structures that are
found on all insects are usually designated as setae (Fig 6); and the flattened, spatulate structures may be
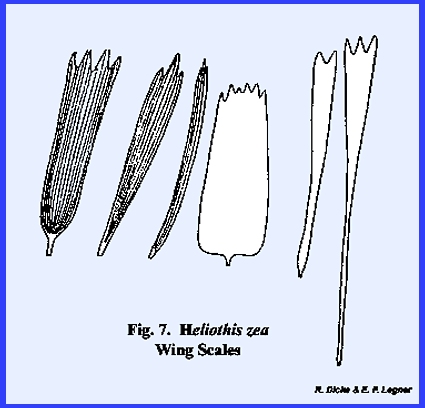
correctly identified as scales (Figs. 7 & 11). All unicellular
processes arise from a well-defined socket and are seated in a flexible
membrane. The socket of a unicellular
process distinguishes these structures from the fixed cuticular microtrichia,
which they frequently resemble.
Unicellular processes may be further modified into sensory and
protective structures. Setae may be
associated with nerve cells and accomplish a tactile or olfactory function. The importance of numerous
sensory structures scattered over the surface of the body is evident when it
is understood that the sclerotized integument effectively isolates the insect
from its environment. A modified
hypodermal cell may secrete an urtication fluid into a hollow setae. When such a seta is broken in the tissues
of a predator, it serves as a deterrent.
Setae may be found profusely scattered or in constant patterns on the
insect's body or appendages wherever cuticular structures occur. They are abundant on the compound eyes of Apis mellifera, on all of the
mouthparts of most insects, on the relatively naked wings of Leucophaea maderae, and on the
external genitalia of Phyllophaga
rugosa. Most setae occurring on
the body probably serve only as a protective covering and as such appear to
be scattered without any particular design.
These may be referred to as secondary
setae. However, certain setae
may be heavily sclerotized and pigmented, and appear bristle‑like and
conspicuously larger than the more numerous secondary setae. These setae, commonly called primary setae, are usually arranged in a constant and
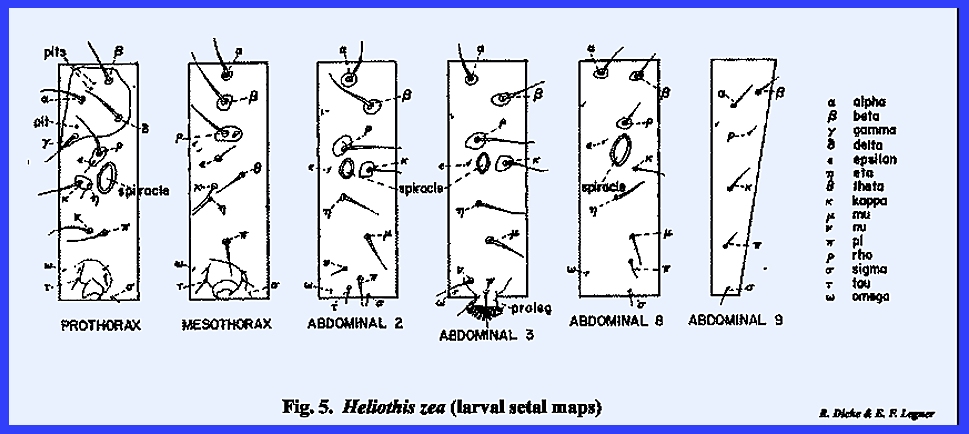
bilaterally symmetrical pattern peculiar to a species (e.g., Fig 5). The setal design or positioning of setae
on the left side of a metamere is a mirror image of the setal arrangement on
the right side. Their arrangement may
be so constant that the design may be employed as taxonomic
characters (Fig
5).
The study of setal arrangements, their use in identifying insect
species, and the nomenclature applied to these setae is known as chaetotaxy. The
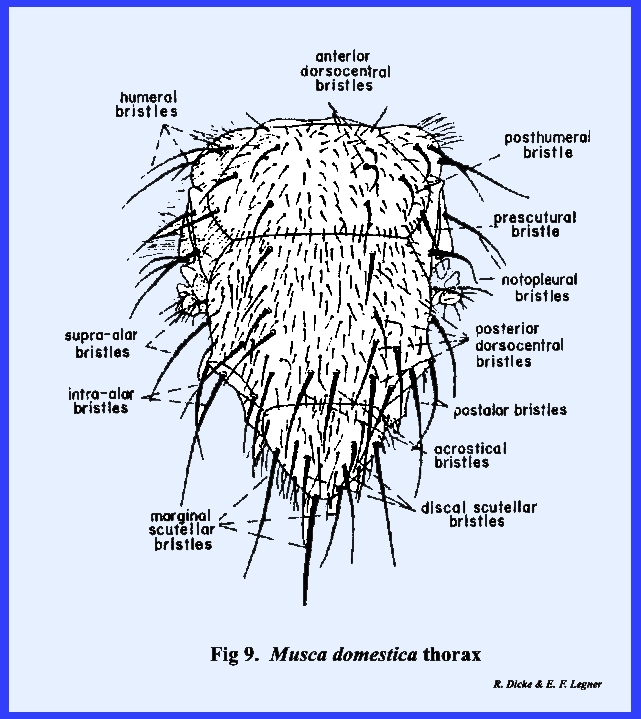
dorsal thoracic setae of Musca
domestica may be used to distinguish primary from secondary setae (Fig
9). The relatively small setae illustrated are
secondary setae. It should be noted
that they are numerous and that they do not occur in a constant pattern. The large conspicuous setae (designated bristles by descriptive entomologists) are differentiated
as primary setae. These setae are
arranged in a bilaterally symmetrical design peculiar to Musca domestica. The
nomenclature employed in chaetotaxy varies considerably from one taxonomic
group to another. Primary setae of
muscoid flies are designated by terms that are descriptive of their position
on the thorax, e.g., anterior dorsocentral bristles (Fig 9) (situated on the anterior sclerite of the thoracic
tergum on more or less a central line), acrostical bristles (setal rows in
parallel lines or across from each other), etc. Chaetotaxy has been extensively employed in the taxonomy of
such naked larvae as the caterpillars of Heliothis
zea (Fig
5).
Comparative arrangements and size of setae are plotted on a
rectangular setal map. The left side of a particular
metamere from the mid‑dorsal to the mid‑ventral line is
included. The positions of the
primary setae in relation to each other are good taxonomic characters since
they are constant for a species but quite variable between species. Primary setae of insect larvae are usually
designated by letters of the Greek alphabet (Fig 5),
although various numeral and/or letter systems are also encountered in the
literature. Setal patterns are not
the same on all of the metameres. The
first thoracic metamere is distinct from the 2nd and 3rd. In Heliothis
zea, one seta, RHO situated above the spiracle, is more prominent than
others since it is usually seated on a raised and distinctly pigmented area (Fig 5). Using RHO as
a central point for Heliothis zea,
it will be noted from the drawing that four prominent setae occur above it on
the first (prothoracic) metamere (ALPHA, BETA, GAMMA, and DELTA). It also occupies a pigmented area with an
additional smaller seta (EPSILON). On
the 2nd & 3rd thoracic metameres (mesothorax and metathorax), two setae
(GAMMA and DELTA) are absent. On the
mesothorax, seta ALPHA lies directly above BETA in comparison to its more
anterior position on the prothorax.
The setal arrangements on the first seven abdominal metameres are
uniform but are not comparable with the thoracic metameres. To illustrate, seta EPSILON lies dorsad of
the spiracle on the prothorax but anterior to the spiracle on the abdominal
metameres. The position and number of setae below the spiracle is also quite
different when a comparison is made of the thoracic and abdominal regions. Abdominal metamere 9 is comparatively
narrow, does not bear a spiracle, and has a reduced setal pattern. Taxonomists usually figure as the most diagnostic, the
first and 2nd thoracic metameres, the 2nd and 3rd abdominal metameres (the
3rd bearing an abdominal appendage, the proleg), the 8th
metamere, and the reduced 9th.
Although secondary setae are arranged in a constant pattern on many
species of insects, occasional variability can be expected. In the thoracic illustration of Musca domestica for example (Fig 9), the 2nd anterior dorsocentral bristle is
absent. The socket in which it was
previously seated may identify a broken seta. However, these should not be confused with naturally occurring
punctures in the cuticula. These
punctures are referred to as pits as illustrated on the
prothorax of Heliothis zea (Fig 4). Pits are usually external openings
associated with chemical sense receptors situated in the cuticula. Tubular, hair like setae are the
more common unicellular protuberances encountered in insects. However, they may be modified into
spatulate or plate‑like structures referred to as scales. These may represent a variety of shapes
from elongated fringe scales to broad plates as illustrated by the wing
scales of Heliothis zea (Fig 7). Body scales are also abundant in some
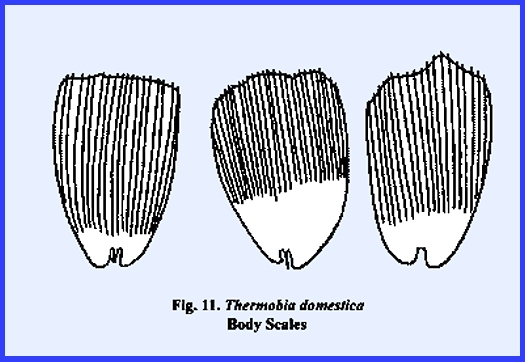
insects as illustrated by the broad thoracic scales of Thermobia domestica (Fig 11). The scales may be pigmented and precisely
arranged in an overlapping pattern comparable to the placement of shingles on
a roof. Parallel ridges that form
minute striations usually mark the flat plane of the scale. This sculpturing of the scale may produce
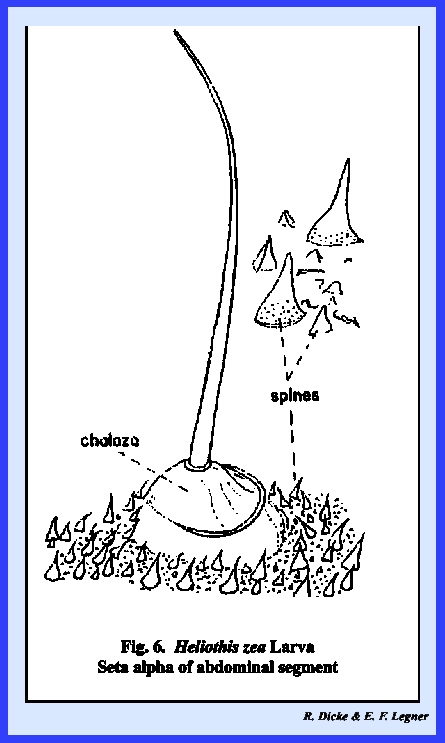
a physical coloration due to interference of reflected light. Protrusions of the entire body wall
including the formative epidermis comprise the relatively conspicuous multicellular processes.
Such a process may be a simple elevation of the integument bearing a
unicellular seta at its apex. The
illustration of seta ALPHA in Heliothis
zea (Fig 6) is an
example of a simple multicellular structure termed a chalaza by
descriptive entomologists. Common
examples of the more conspicuous multicellular processes are the heavily
sclerotized, spiny structures termed spurs that are encountered
on the legs of many insects. These
spurs may be fixed and confluent with the cuticula. Others may be set in a membranous ring and are therefore
movable as illustrated by the tibial spurs of Leucophaea maderae (Fig 8).
Multicellular processes may bear fixed spines as the microtrichia on
the spurs of Leucophaea maderae (Fig 8) as well as single or numerous unicellular setae. = = = = = = = =
= = = = = = = = = = = SECTION II ‑ THE HEAD
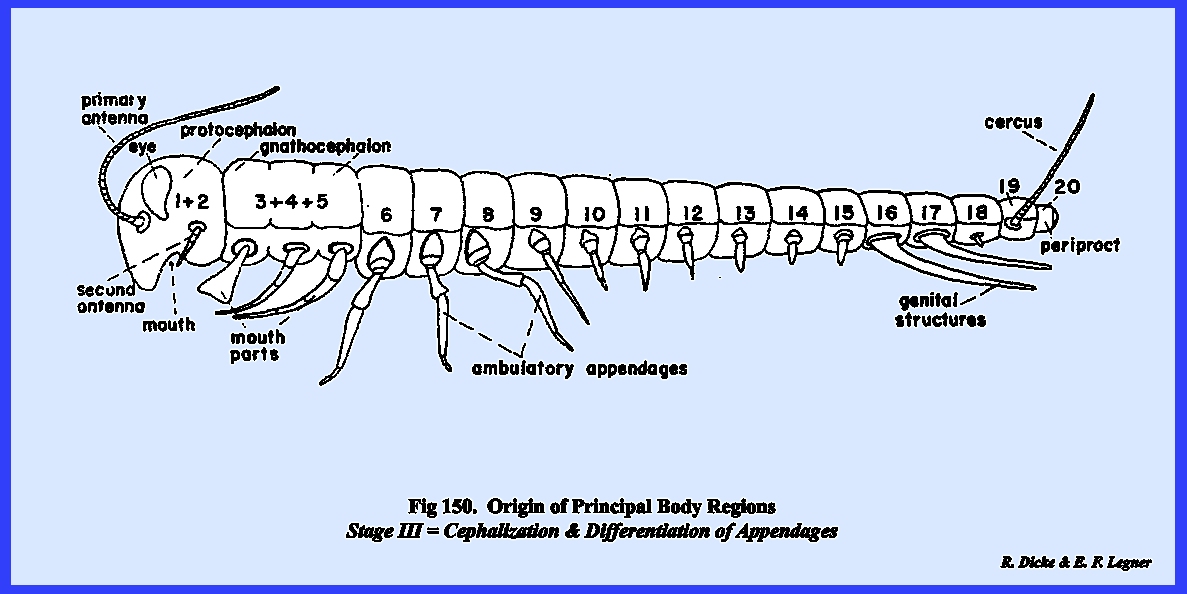
Evolution
of the Insect Head
The principal regions of the
insect body are thought to have evolved as composites of cylindrical
metameres, each of which in the primitive form bore a pair of ambulatory
appendages./1 (See Figs.
148-151): While this theory seems plausible
for the abdomen and in most forms for the thorax, it appears at first
examination to be a rather remote assumption for the head region. The head capsule has become a highly
evolved or specialized structure involving at
least five primitive or generalized
metameres. The first metamere or prostomium probably bore the mouth opening at its
posterior margin in addition to a pair of appendages that evolved into the sensory antennae.
A study of the brain of present‑day insects and the head region
of certain related arthropod forms such as the Crustacea has led
morphologists to assume that the prostomium and the next following metamere
(first postoral) both developed sensory antennae. With later evolution, the principal sensory structures were
then situated on the first two metameres.
These metameres may have fused early in the evolution of the head to
form a theoretical protocephalon. The development of the photo receptors or eyes is not
clear, although these sensory structures are believed to have developed on
the prostomium. From a comparative
study of the morphology of present‑day insect mouthparts and the nerve
centers associated with them, it may be concluded that these organs of
ingestion probably evolved from ambulatory appendages. Since three pairs of structures make up
the generalized feeding mechanism, it may be assumed that three metameres
were involved in the formation of a second primitive head complex or gnathocephalon.
In the present‑day insect, the sensory protocephalon and the
ingestive gnathocephalon have coalesced and have become completely fused into
a composite structure. Unlike the
thorax and abdomen, segmentation of the head is obscure and the sutures as we
know them today have little correlation with the metameres that were involved
in its formation. The
Typical or Generalized Insect Head
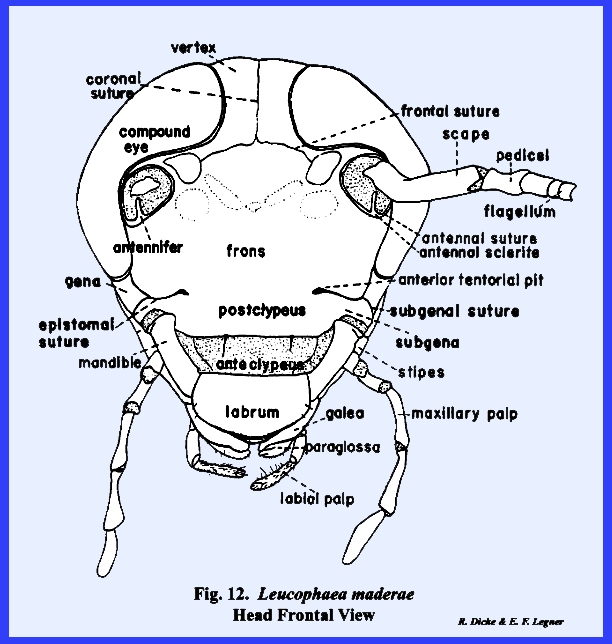
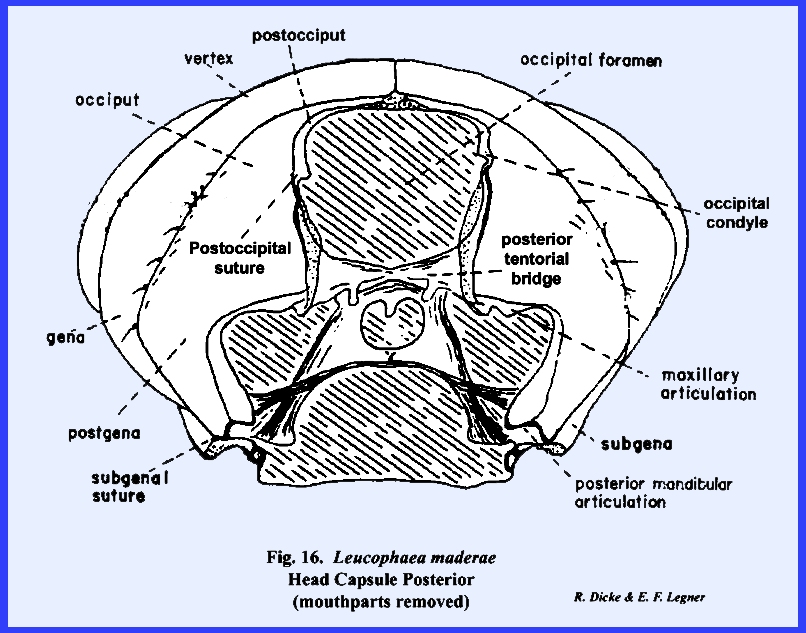
The head of Leucophaea maderae may be used to illustrate a typical,
generalized form of head capsule (Figs.
12-16):
Essentially, the head is an ovoid envelope of sclerotized integument
enclosing the brain centers, certain glands, and muscle systems for the
operation of the head appendages. The
head capsule is open at its posterior juncture with the thorax to permit a
passageway for certain connectives such as the ingestive tube, which connects
the mouth with the digestive system.
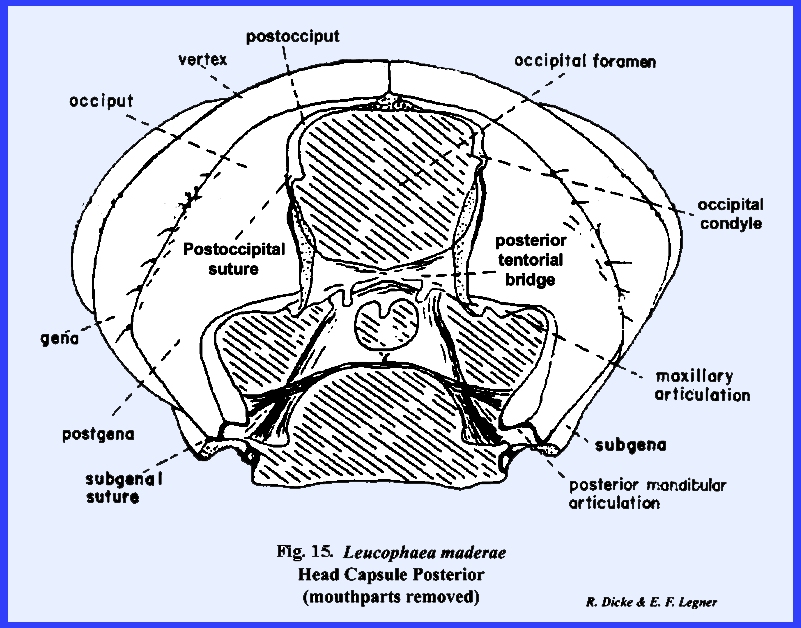
This opening is called the occipital
foramen. The thin, flexible
cylinder of integument connecting the margins of the occipital foramen with
the thorax is the neck or cervix.
A mouth opening is situated on the ventral aspect of the capsule that
is also depressed to form a pocket or oral cavity to
accommodate the operation of the mouthparts. Internally, an A‑shaped,
composite apodeme formed by invaginations of the integument, braces the head
capsule before the oral cavity. This
brace is the tentorium, and the points of invagination of the integument are
the tentorial pits. Usually, the
tentorium is well developed in insects that have powerful biting and chewing
mouthparts to form an internal strut, to prevent the moving jaws from collapsing
the head capsule. In Leucophaea maderae, the anterior
invaginations or anterior tentorial
arms unite mesally to form a bridge,
while the posterior invaginations form at the base of the occipital foramen
a posterior tentorial bridge
(Figs 15 & 16). The fused anterior
tentorial arms and posterior tentorial bridge are united into a common, A‑shaped
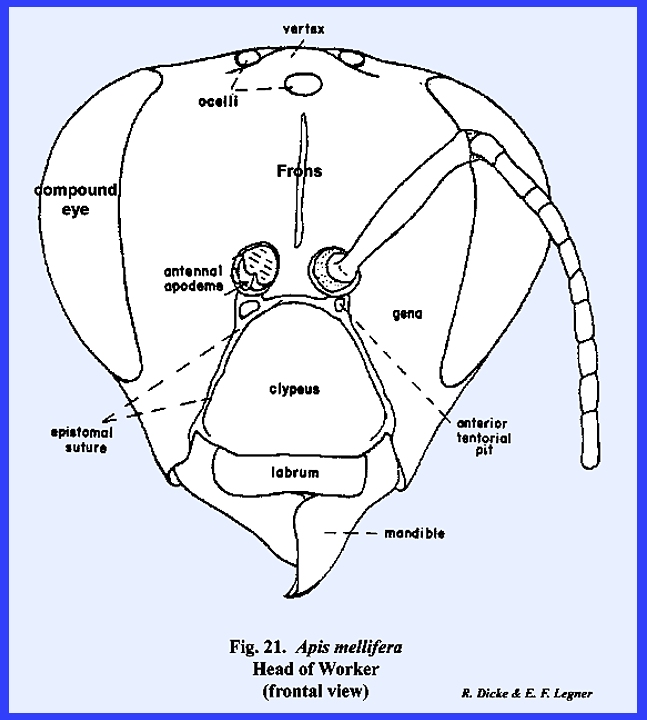
structure leaving a median opening for the passage of nerve trunks. The conspicuous photoreceptors or compound eyes occupy the dorso‑lateral aspects of
the head, and the antennal sockets are situated on the frontal surface
between the eyes. A suture outlines
and separates the compound eye and antennal socket from the adjoining
sclerotized areas. These sutures may
also enclose a sclerotized area forming a ring about the sensory
structure. In Leucophaea maderae, there is an ocular
suture enclosing an ocular sclerite (Fig 13), and an antennal suture enclosing an antennal
sclerite (Fig 12). The anterior surface of the head lying
between the compound eyes is designated as the frons (Fig 12). Although the frons is usually easily
identified as the broad frontal area between the eyes, an accurate
identification of facial areas is best made with reference to the sutures
lining the integument of the head. It
should be emphasized that while certain head sutures are relatively constant
in position, they do not represent the primordial divisions of the metameres
that originally formed the head region.
Ventrad of the frons in Leucophaea maderae is a short suture
bearing at its mesal ends the anterior
tentorial pits. This is the epistomal suture (Figs 12 & 13). In most insects,
the epistomal suture is continuous across the face and is probably the most
constant frontal suture to use for the identification of facial areas. The anterior arms of the tentorium are
usually anchored on the apodeme or an epistomal
ridge formed by the invagination of this suture. When anterior tentorial pits are present,
they will always be found on the epistomal suture. If the anterior pits are not developed, the suture may be
identified by dissection of the head that may reveal that the tentorial arms
are anchored on the epistomal ridge.
In some species, the tentorial pits are readily identified, but the
epistomal suture is absent, or incompletely developed as in Leucophaea maderae. An imaginary line drawn between the two
pits will represent the absent suture and will serve to identify the facial
areas usually separated by it. The
facial area above the epistomal suture is the frons; the area below the
suture is the clypeus.
Occasionally, the distal portion of the clypeus is
membranous. The proximal sclerotized
portion of the clypeus is then identified as the postclypeus
and the distal, membranous portion as the anteclypeus
(Fig 12). An oblong sclerite freely articulating at
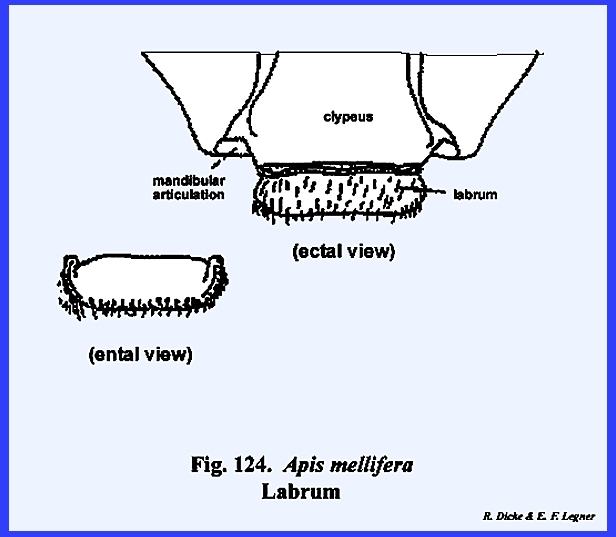
its proximal margin with the clypeus, is the labrum. This sclerite serves as an upper lip for
the mouth cavity. Although the labrum is generally
considered as a part of the organs of ingestion, it is a true sclerite of the
head and was not evolved from an appendicular structure. The gena or cheek
is a poorly defined area in most insects, but usually lies below and
immediately behind the compound eyes.
In Leucophaea maderae, this
area is set off by a short subocular groove (Fig 13). An area immediately above the
articulations of the mandibles may be heavily
sclerotized to support the powerful jaws.
This area margined by a subgenal
suture is designated as the subgena.
The subgenal suture is usually continuous with the epistomal
suture. A frontal suture resembling
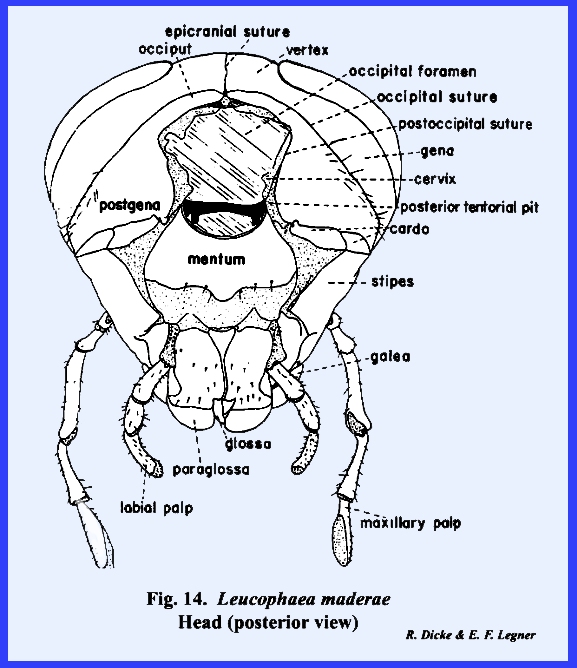
an inverted Y is common in immature insects and is known as the epicranial suture.
This is actually an ecdysial suture or a point of
rupture in the integument during the molting process. The epicranial suture is uncommon in adult
forms, although it is faintly represented in Leucophaea maderae (Fig
14). The stem of
the Y is referred to as the coronal suture and the
arms as the frontal sutures. When this suture is developed, the area
enclosed by the frontal sutures is designated as the frons. The top of the head as a poorly defined area is the vertex. When an
epicranial suture is present, the vertex is the area immediately to either
side of the coronal suture.
Identification of the posterior areas of the head is best accomplished
by locating the posterior tentorial pits (Fig 14). These mark the point of invagination of
the posterior tentorial bridge. The
pits are always situated on a postoccipital
suture. As for the epistomal
suture in the frontal region, the postoccipital suture is usually the most
constant suture of the posterior region.
The sclerite enclosed by the postoccipital suture is the postocciput which serves as a sclerotized ring about the
occipital foramen. The neck membrane
or cervix is attached to this sclerite, and a mesal projection or occipital condyle serves as a point of articulation
for the sclerites of the cervix. An ---------------------------------------------------- 1/
Refer to Section IV ‑ Origin
of the Principal Body Regions. additional
suture may occur anteriorly to the postocciput and margins the flat posterior
aspect of the head. In Leucophaea maderae this suture is more
of a marginal ridge, but it may be referred to as the occipital suture and the area enclosed by it as the occiput. Usually, the term occiput is used only to
describe the posterior area immediately behind the vertex. The lateral, ventral portion of this
sclerite is then referred to as the postgena. However, technically the entire sclerite
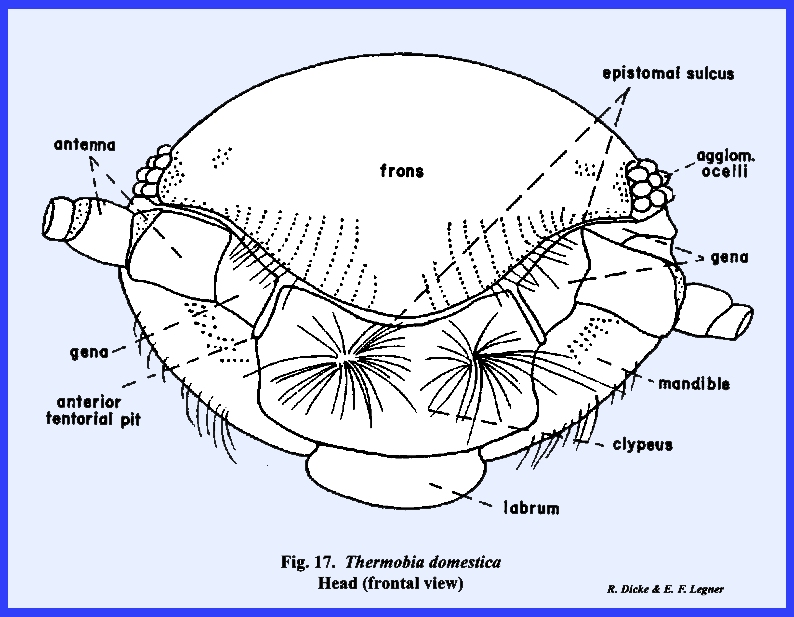
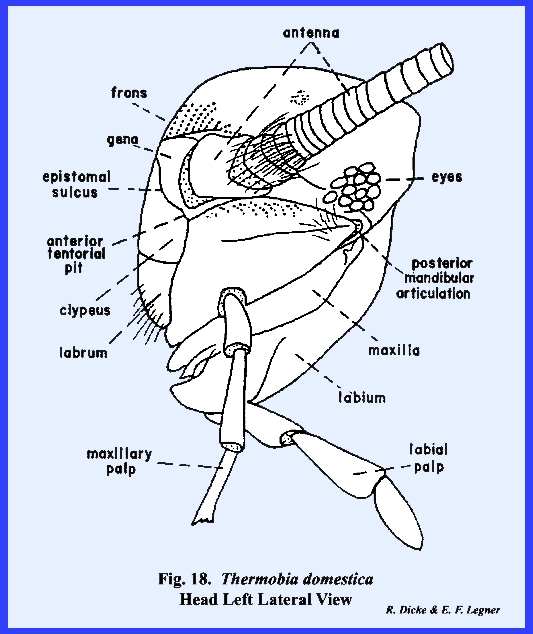
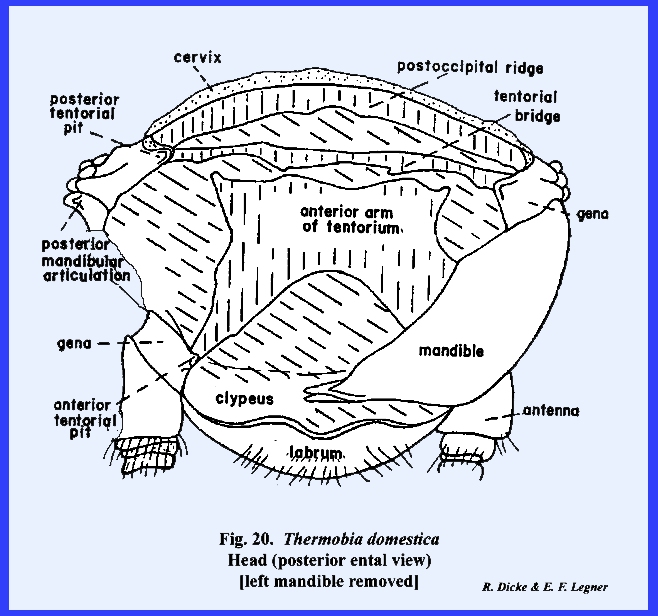
may be correctly referred to as the occiput. THERMOBIA DOMESTICA: The head of Leucophaea maderae was described as the "typical form.” But this does not imply that the head of Leucophaea maderae is primitive in the
sense of being but little elaborated in comparison with a hypothetical
prototype. Thermobia domestica is a relatively primitive insect compared
with Leucophaea maderae. The conspicuous epistomal sulcus of Thermobia domestica will readily
distinguish the facial areas (Figs 17 & 18). Note that the frons and clypeus are large,
well-defined sclerites. The gena,
however, is a small area immediately before the antenna and below the eyes. All of the other head sclerites described
for Leucophaea maderae are
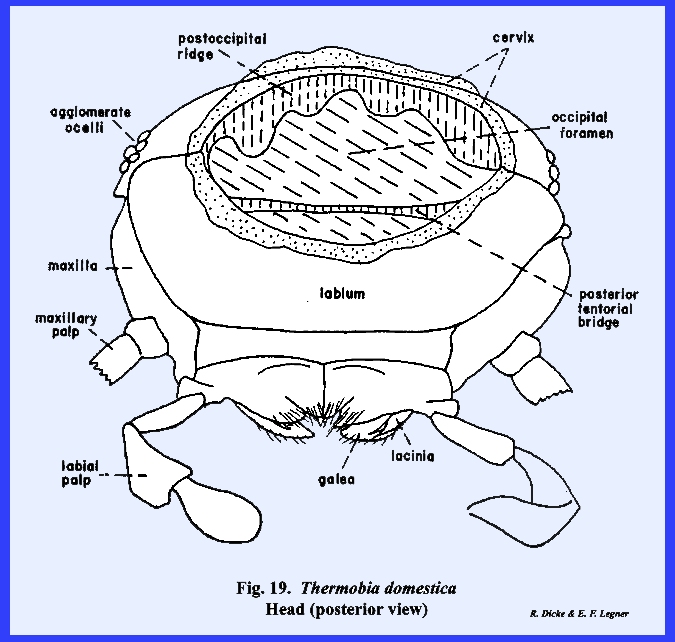
absent. The postocciput as a sclerite
is inconspicuous, but the invagination of the postoccipital suture forms a
large apodeme or postoccipital
ridge (Figs 19 & 20). The tentorium of Thermobia domestica is of special interest to the
morphologist. Previously, this was
defined as a cranial brace formed by the fusion of two anterior and two
posterior invaginations of the exoskeleton forming the head capsule. In Leucophaea
maderae, the tentorium forms an A-shaped structure comprising a posterior
tentorial bridge and two anterior arms.
However, the posterior tentorial bridge of Thermobia domestica has not fused with the anterior arms although
a large central plate has been formed by the posterior fusion of the anterior
arms. If the theory on the formation
of the tentorium is correct, it may also be assumed that in Thermobia domestica this is a
relatively primitive structure. Specializations
in the Adult Head Structure
Further modifications of the insect head from the typical form
may occur in 1) the fronto‑clypeal region, and 2) the posterio‑ventral
region. For many of the highly
evolved forms, these modifications may progress to the point where it is
difficult, and in some forms impossible to compare or homologize the
sclerites with the typical form. This
is especially evident in species that have evolved highly specialized sucking
mouthparts, or in the larvae of immature forms of the Endopterygota. Where the structures cannot be identified,
it may then be necessary to borrow a descriptive term from the taxonomic
literature. When the epistomal suture
is intact, there is little difficulty in identifying the facial sclerites. The area above the suture is the frons, and
the sclerite below is the clypeus.
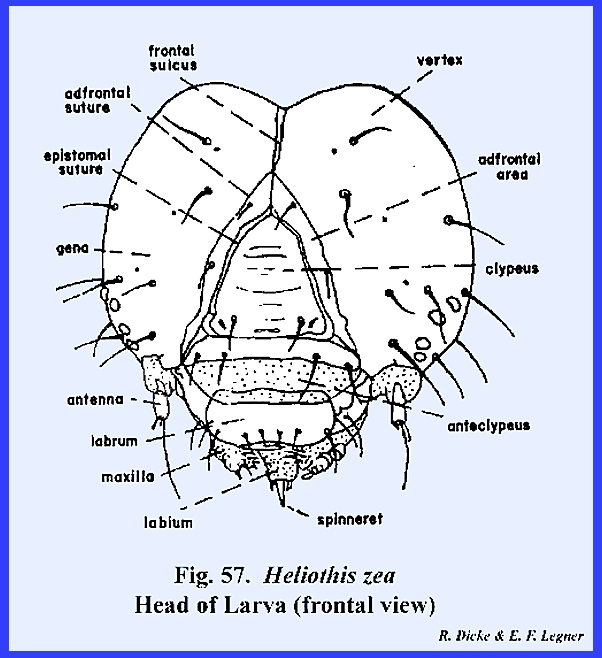
The epistomal suture is not always in a transverse line. In the adult of Apis mellifera (Fig 27) and the
larva of Heliothis zea (Fig 57), this suture
is strongly arched dorsad and resembles the epicranial suture. Since the tentorial pits are situated on
the suture, the area enclosed by it would resemble the frons but would be
incorrectly identified as such. In
the absence of the epistomal suture, the tentorial pits may determine the
relative areas since the anterior arms of the tentorium are always anchored
in position on the epistomal ridge.
Dissection of the head will also determine the position of the tentorial
invagination should the pits be indistinct.
Certain muscles of the sucking apparatus and ingestive canal arise
from either the frons or the clypeus, and these sclerites can be identified
by their muscular attachments. Where
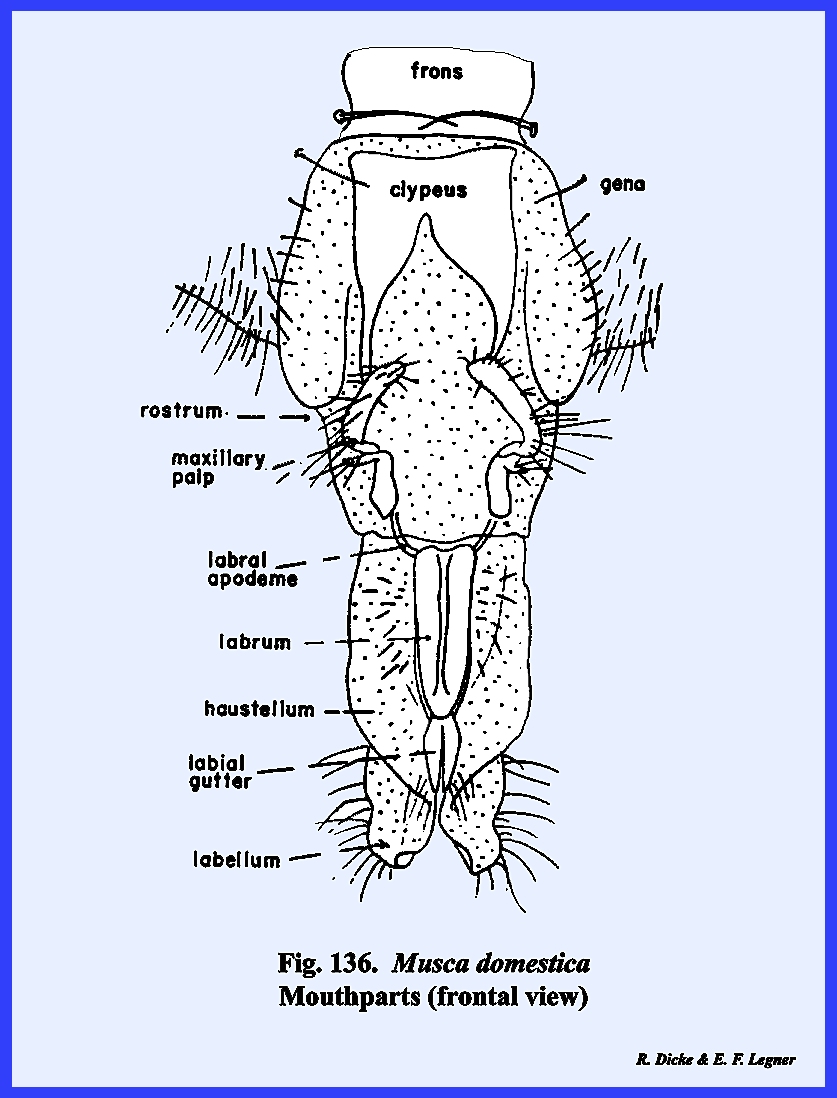
the tentorial arms are greatly modified or where they are absent as in Musca domestica, a study of the
musculature of the sucking apparatus is the only clue to identification. The posterio‑ventral aspects
of the head are modified in many forms so that the mouthparts may project
forward. In the generalized form, the facial area is directed
forward and is anterior and vertical in position. The mouthparts are pendant or hang ventrally in position, and
the labium that forms the floor of the oral cavity is attached to
the cervix. This position of the head
is referred to as the hypognathous form. Direction of the mouthparts forward is
advantageous to many species. The
head is rotated upward with the mouthparts directed anteriorly, and the
facial region is now in a relative horizontal or dorsal position. This modification is known as the prognathous form.
In order that the occipital foramen will retain its vertical plane,
the ventral surface o, the head must be elongated. This is accomplished by 1) the formation of a gula that is
a sclerotization of the neck membrane at the base of the labium, and 2) by a
lateral expansion of the subgenae.
The expanded postoccipital suture always encloses the gula. When a gula is present, the postoccipital
suture is often referred to in descriptive literature as the gular suture. As
the ventral aspects of the head are expanded in the prognathous form, the
attachment of the labium becomes further removed from its original attachment
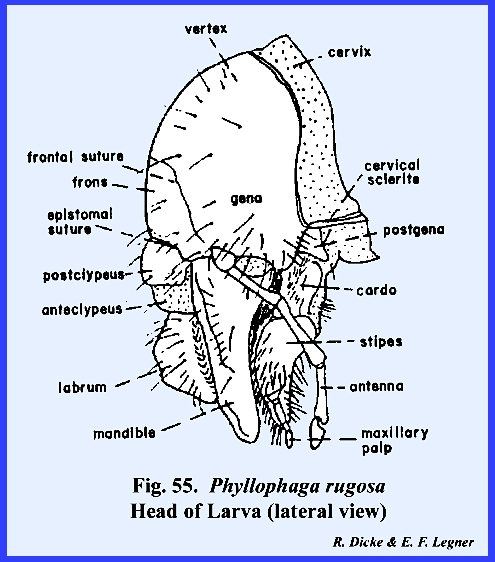
to the cervix. PHYLLOPHAGA RUGOSA (Fig
3). The head capsule of Phyllophaga
rugosa is oval in shape, flattened dorso‑ventrally, and the facial
area is essentially like that of the typical form. It is heavily sclerotized and further strengthened by a TT‑shaped
tentorium. The posterior tentorial
bridge is weak, but the anterior arms are well developed and have become
fused with the ventral sclerites.
Posterior tentorial pits lying on the gular suture are well developed,
but the anterior pits at the base of the compound eyes are difficult to
demonstrate. However, the anterior
tentorial arms are attached at the outer margin of the epistomal suture. Unlike Leucophaea
maderae, a well-developed gula has projected the mouthparts forward. The head of Phyllophaga rugosa is therefore of the prognathous form. Two other modifications distinguish this
species from the typical form: the clypeus is strongly reflexed to produce a
ledge which overhangs the labrum, and a slender sclerite given the
descriptive term of canthus projects into the ocular region (Fig
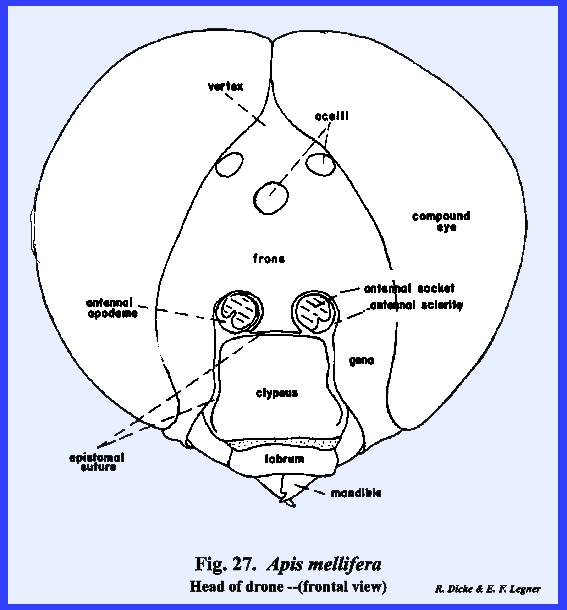
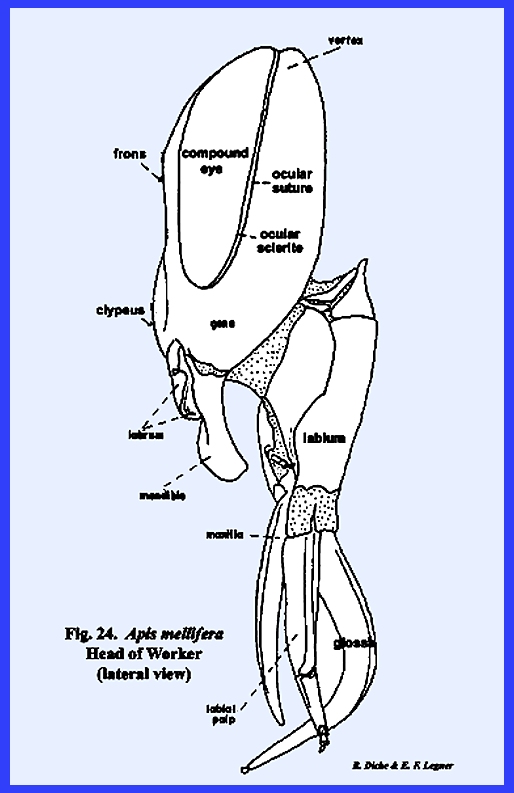
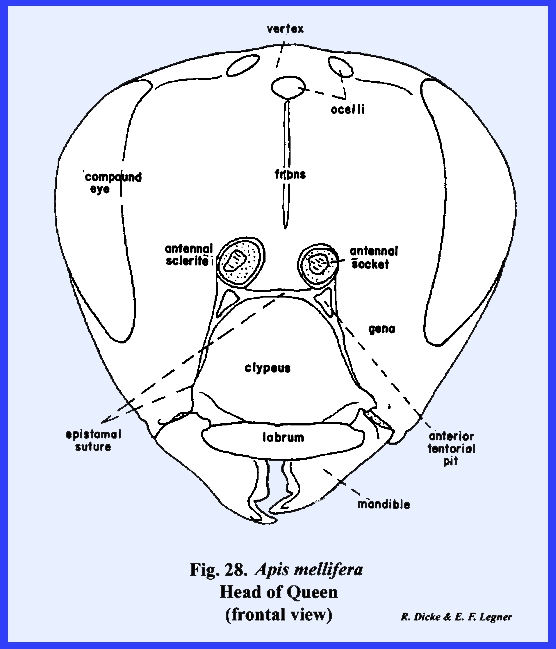
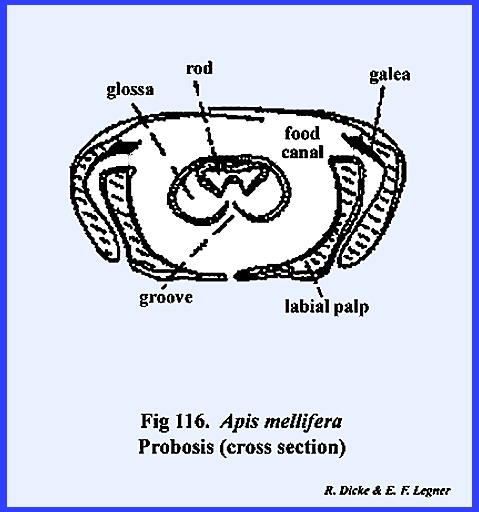
3). APIS MELLIFERA: At first examination, the head of Apis mellifera appears like the
typical form previously described including a "typical" epicranial
suture. It was already noted that the
epistomal suture sometimes is strongly arched upward enclosing a triangular
sclerite that is often incorrectly identified as the frons. This is definitely the epistomal suture
since the anterior tentorial pits are situated on it at a point below the
antennae. Therefore, the area
enclosed by this suture is the clypeus (\). Unlike Leucophaea maderae, the antennae are
considerably removed from the margins of the compound eyes, and a cluster of
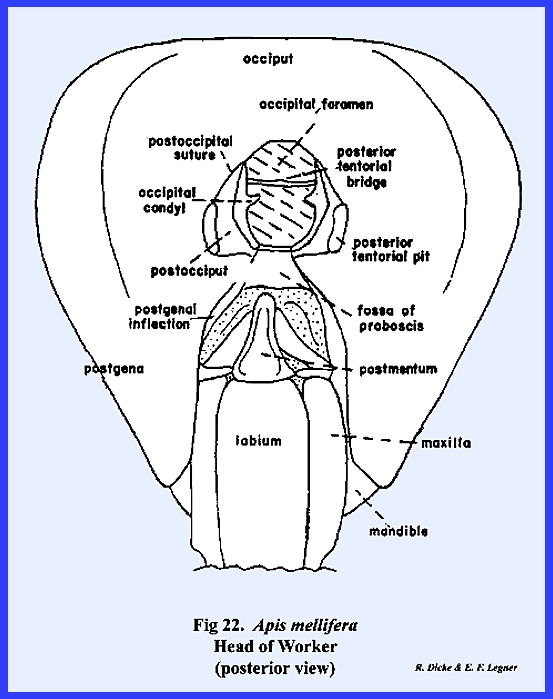
three simple eyes (the ocelli) is situated on the vertex. Posteriorly, the occipital foramen is
greatly reduced in size compared with Leucophaea
maderae or Phyllophaga rugosa,
an occiput is not clearly defined, and the postocciput is a pair of small
sclerites on either side of the foramen.
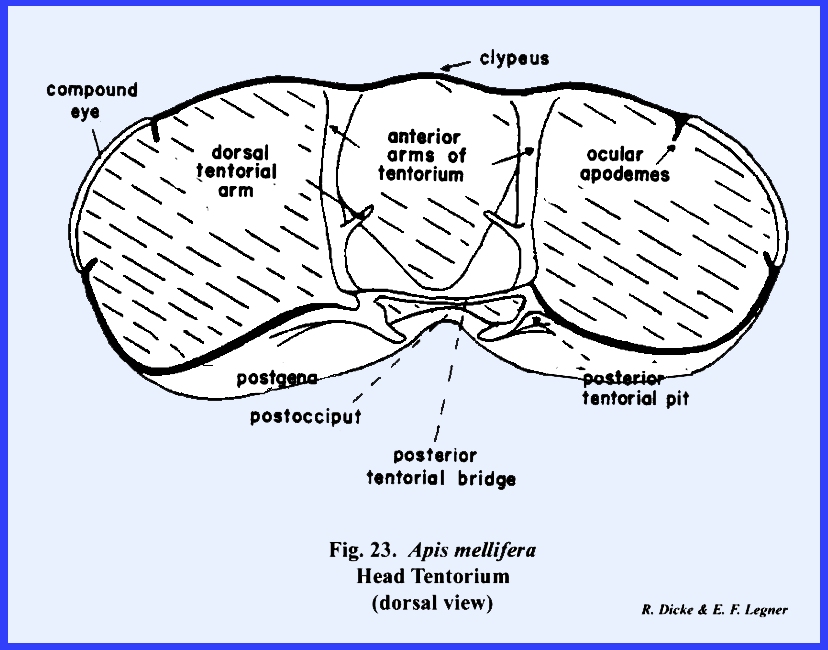
These are clearly identified by the posterior tentorial pits. On the ventral aspect of the head, the
postgena has become deeply invaginated to form a pocket within which the base
of the mouthparts is seated (Figs 22 & 24). This pocket may be
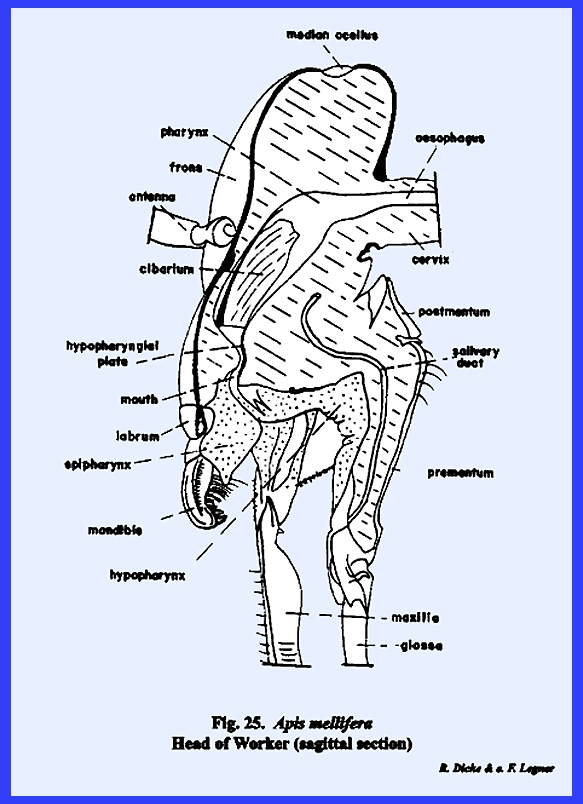
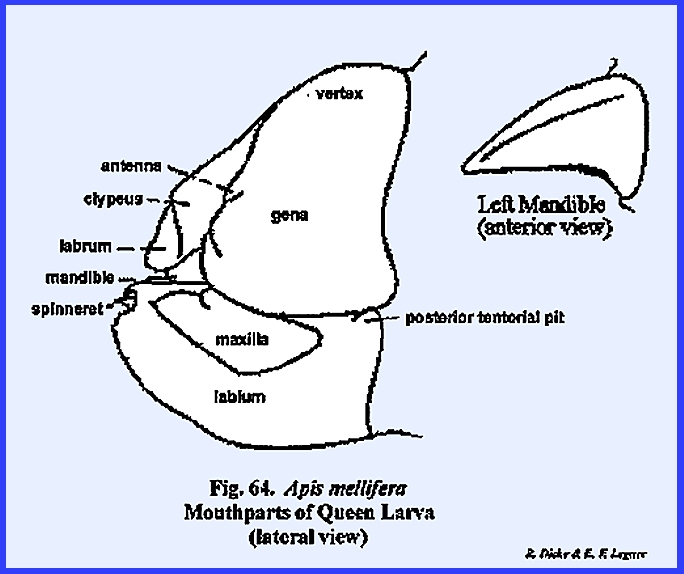
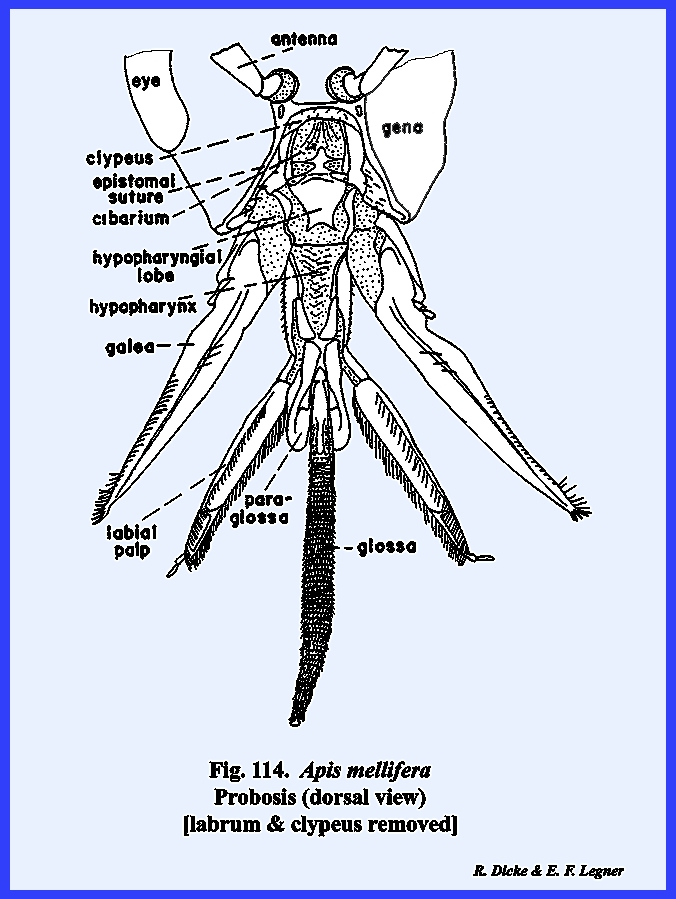
referred to as the postgenal inflection. The mouthparts of Apis mellifera will be discussed in
considerable detail in the following section III, but it should be noted at
this point that the mouthparts of the typical chewing form have been modified
into a complex sucking mechanism.
However, the mandibles have been retained as functional structures
comparable to those of Leucophaea
maderae and Phyllophaga rugosa. The tentorium is a typical TT‑shaped
brace with a posterior tentorial bridge and strong anterior arms. A sexual
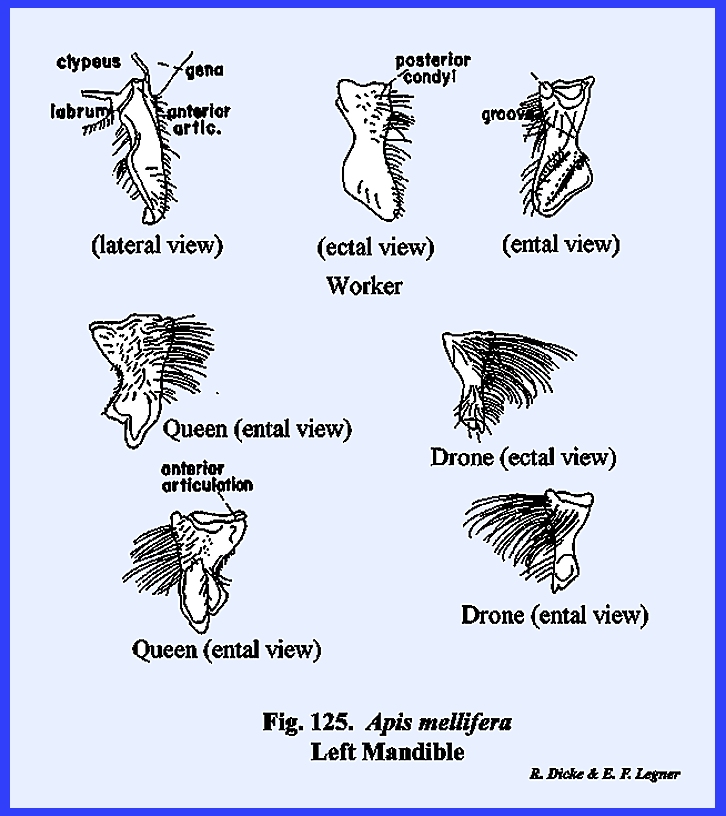
dimorphism is very evident in the head of Apis mellifera. The heads
of the queen and the worker (in which the sexual organs are retarded) are
comparable in form (Figs 21 & 28). In the male, or
drone, the compound eyes are greatly expanded at the expense of the frons and
gena, giving the head an appearance that at first would seem quite unlike
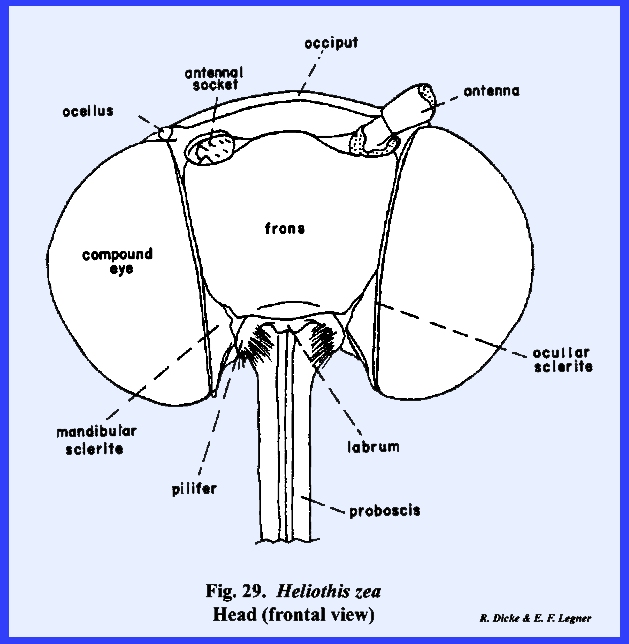
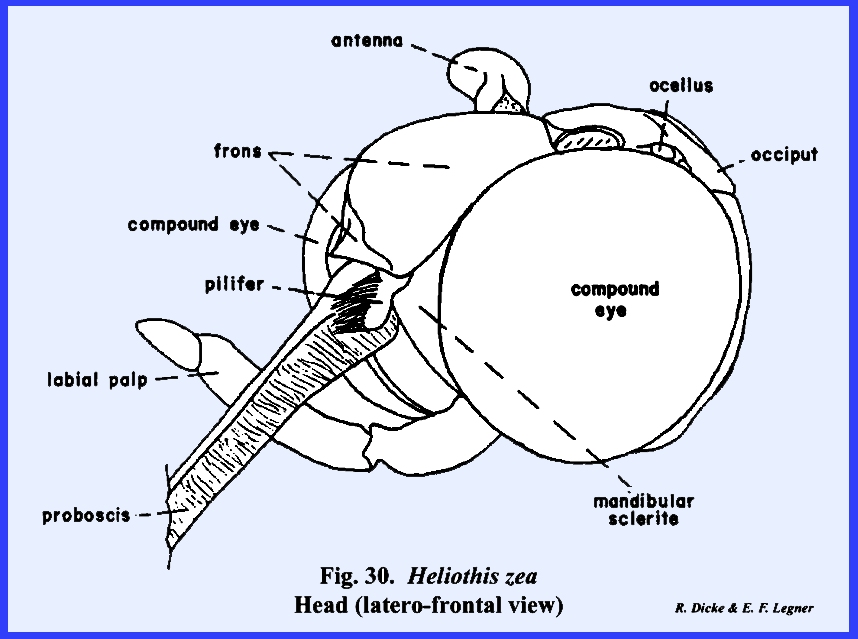
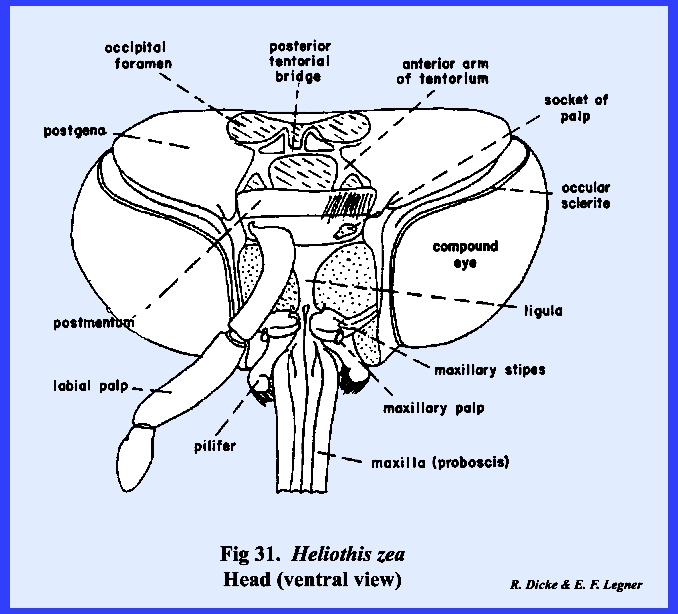
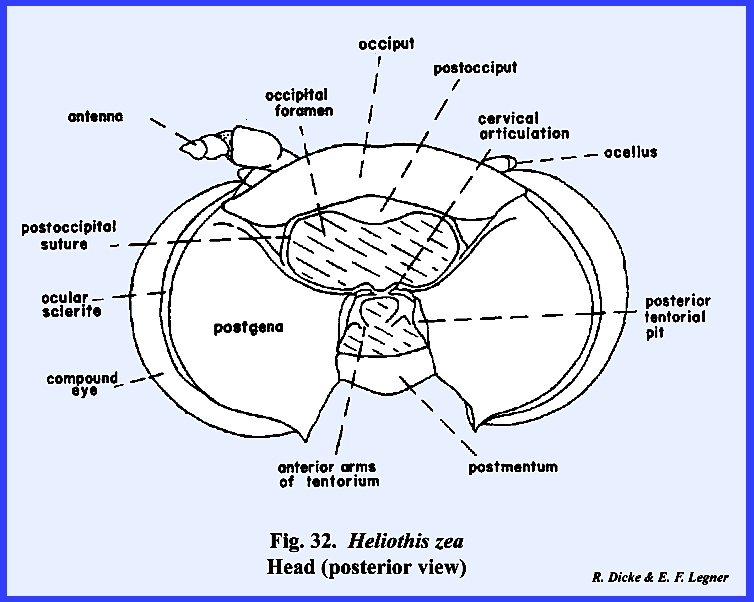
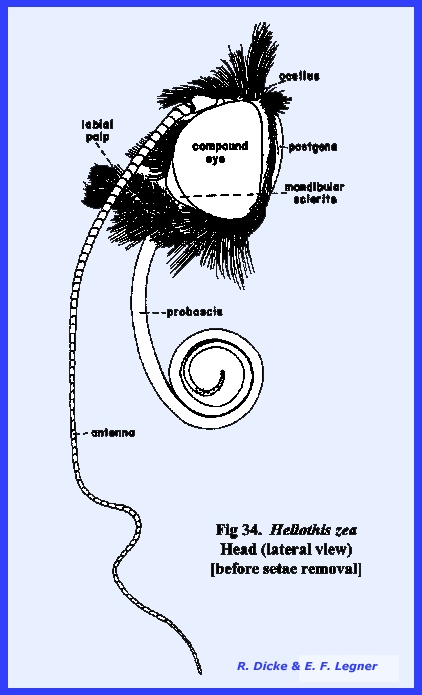
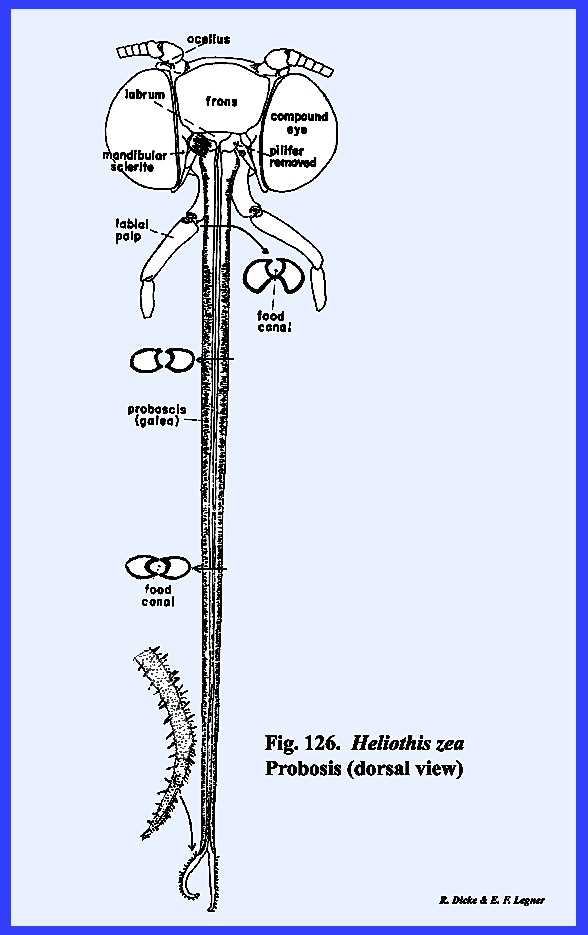
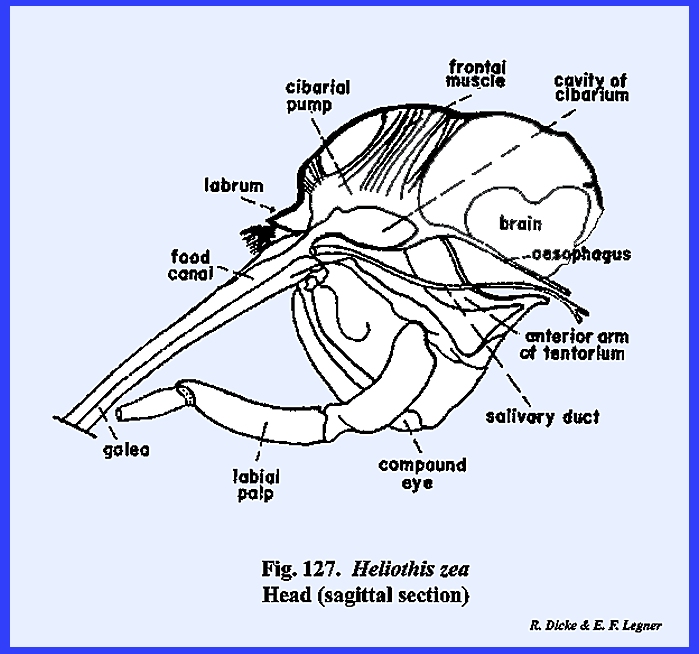
that of the female sex (Fig 27). HELIOTHIS ZEA: The head of Heliothis zea is densely covered with setae, and is conspicuous
for its large compound eves which occupy much of the head surface, long
antennae, and a coiled sucking tube or proboscis
(Fig 34). When the head is denuded of its setae,
only the frons remain of the facial sclerites. The epistomal suture and the anterior tentorial pits are
absent, but the anterior arms of the tentorium are anchored at the posterior
margin of the facial sclerite correctly identifying it as the frons. The gena appears to be absent, although
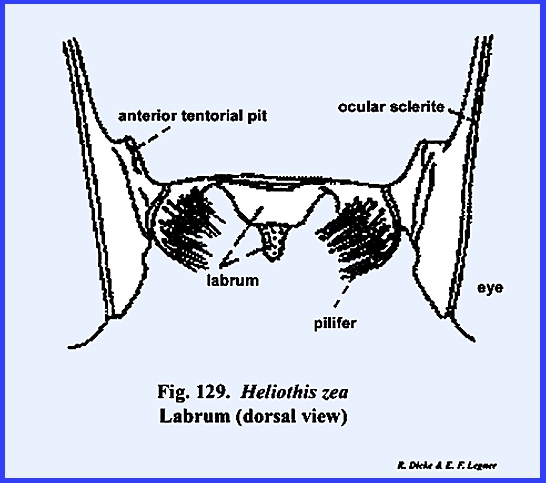
this may be the area described by taxonomists as the mandibular sclerite (Fig 30). The labrum is greatly reduced to an
inconspicuous flap. On either side of
the labrum are two small sclerites given the descriptive term of pilifers (Fig
31). These
sclerites are of unknown morphological origin although they are said to be
remnants of mandibles. Two simple eyes
or ocelli are situated between the antennae and dorsal margin of the compound
eyes. Posteriorly, a dorsal sclerite
appears to be the occiput. A small
postocciput identified by the posterior tentorial pits occurs above and rings
the occipital foramen. Postgenal
sclerites make up the flat, lateral and ventral aspects of the posterior head
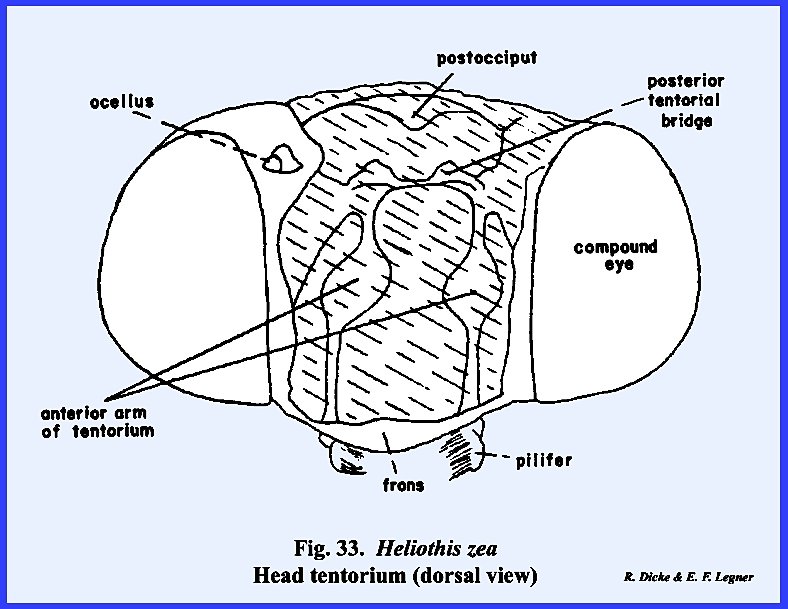
capsule. The tentorium is a typical
TT‑shaped structure, although the posterior bridge and anterior arms
are weak. Of special interest is that
the anterior arms of the tentorium are inflated midway into weakly
sclerotized bulbular structures. The
function of these expansions is unknown.
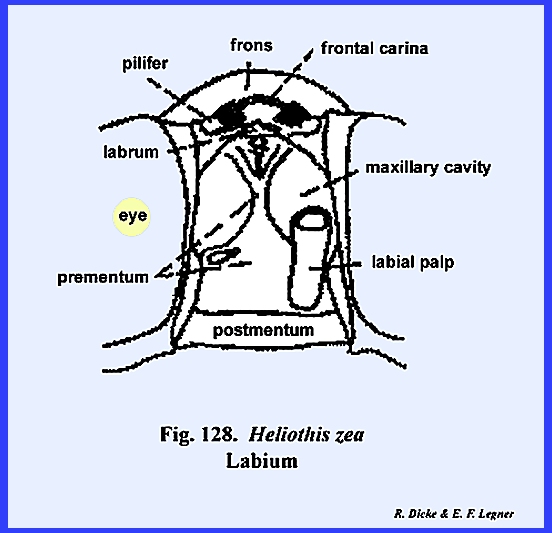
The remaining identified sclerites of the head such as the postmentum
and ligula and appendages such as the proboscis and palps are modified from
and associated with the organs of ingestion and will be discussed in the
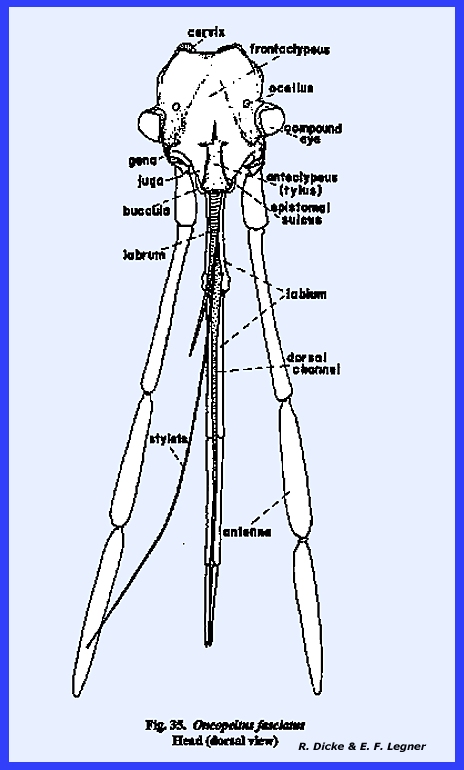
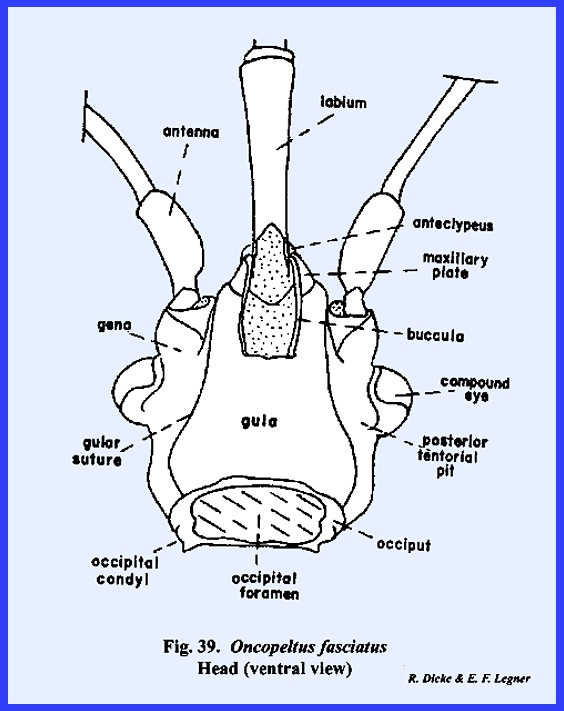
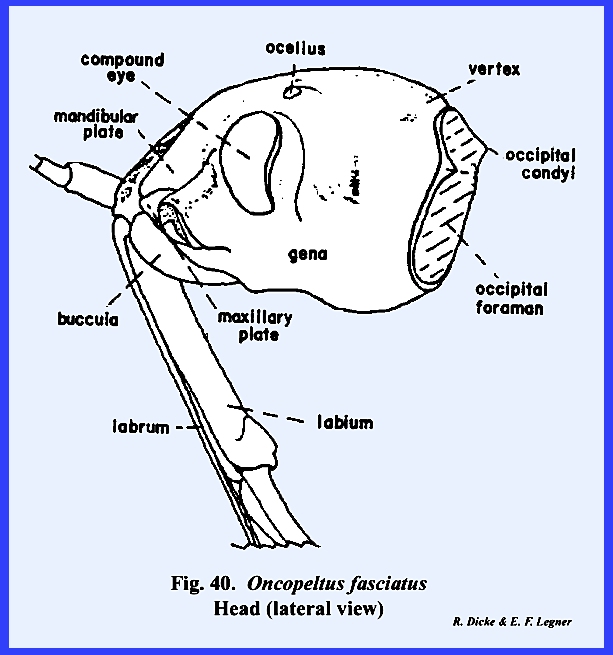
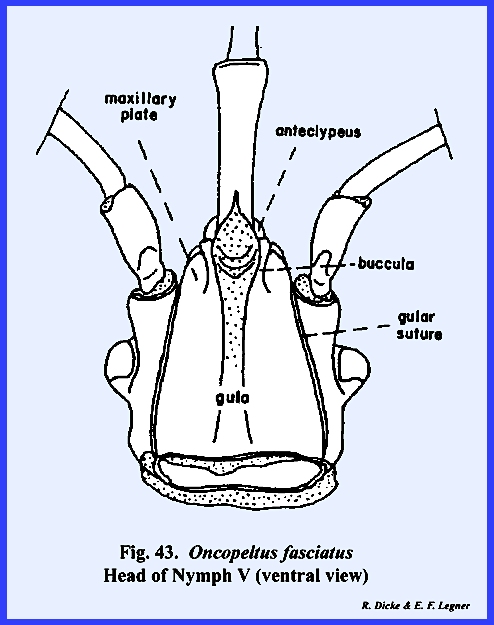
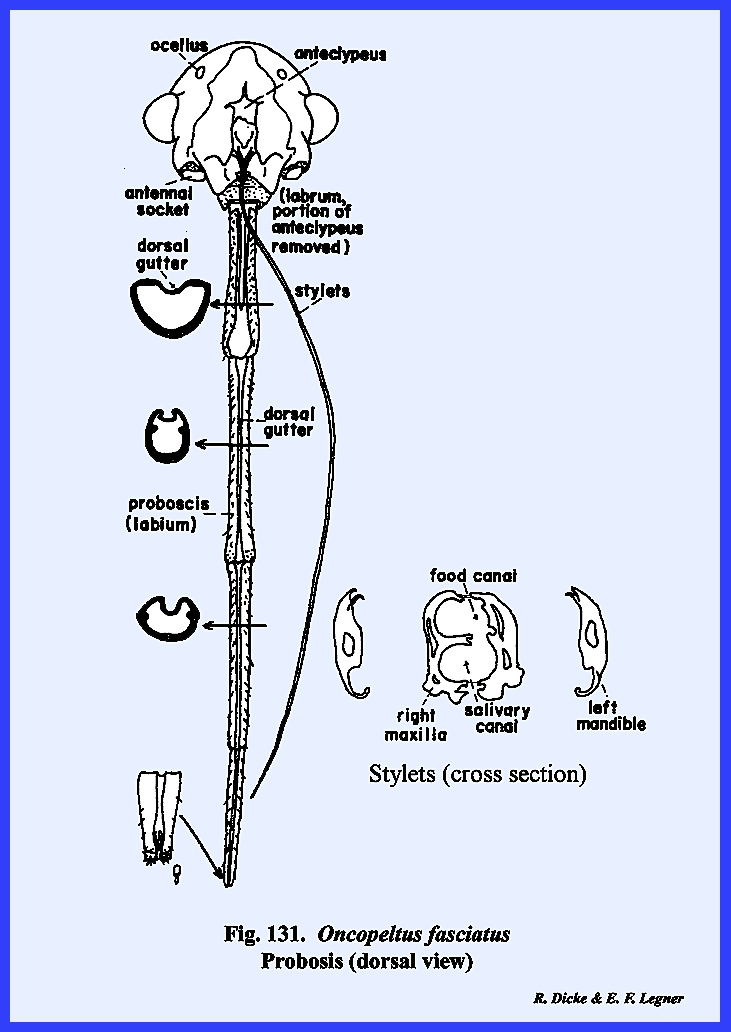
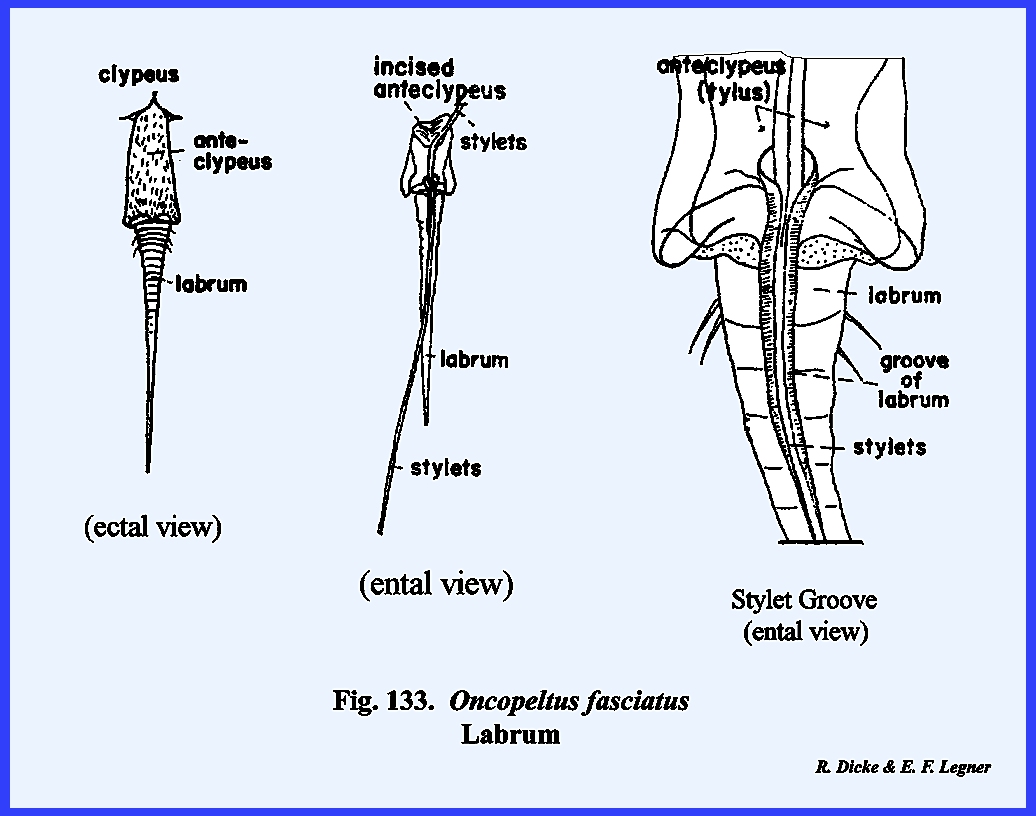
following section. Oncopeltus
fasciatus : Although taxonomists have placed Oncopeltus fasciatus relatively low on
the phylogenetic scale, it is actually a highly evolved form. The organs of ingestion are an efficient
piercing‑sucking apparatus; the head has been rotated forward by the
development of an extensive gula, and the facial sclerites associated with
the mouthparts have been modified to the extent that it is difficult to
homologize many of them with the typical form. An oblong sclerite given the descriptive name of tylus by
specialists of Heteroptera is probably the anteclypeus (Fig 42). This sclerite is confluent with the
integument of the head capsule at its posterior end and is laterally margined
by a deep sulcus, which is probably the epistomal suture. That this sulcus is the epistomal suture
may be assumed since the anterior arms of the tentorium are anchored on the
walls of this inflection. Actually,
this is not a suture in the sense that it is an invagination between two
sclerites, viz., the clypeus and the frons.
The lateral margins of the tylus are not united with the head capsule
and the entire sclerite is fixed only at its posterior end, and lies freely
in a groove formed by the inflection of the integument, which was tentatively
identified as the epistomal sulcus.
The sclerotized walls and partial floor of this groove (best seen by
removing the anteclypeus) is identified as the maxillary
plate (Figs 37 & 43). The two plates or sclerotic areas lying
between the anteclypeus and the base of the antenna are probably an
expansion of the gena. But, this area
has been given the descriptive name of jugum (Fig 42). Since the muscles and apodemes associated
with the mouthparts are also associated with this sclerite, morphologists
have referred to this area as the mandibular
plate (Fig 40). Pigmentation of the head of Oncopeltus fasciatus is such that a
light, triangular area is formed on the facial region. Demarcation of the black pigmentation in
the adult is so distinct that some specialists have assumed the presence of
an epicranial suture, and have named the light triangular area the frons (Fig 42). A distinct epicranial or ecdysial suture
does occur in the immature form or nymph, but
there is no evidence of such a suture in the adult. The dorsal surface of the head (or facial area since this is a
prognathous head) is the frontoclypeus (Fig 35). Later it will be shown that the muscles,
which operate the highly evolved sucking pumps, are anchored on the facial
sclerites. The origin of these
muscles in Oncopeltus fasciatus
indicates that both sclerites are present.
Modification of the head has been such that an epistomal suture does
not separate them, and the entire area must be identified by this composite
term. The compound eyes protrude from
the head capsule by expansion of the genae.
Two simple eyes occur at the bases of the large compound eyes. In Oncopeltus
fasciatus, the labrum is not a simple oblong upper lip as in Leucophaea maderae, but has been
modified into a sharply tapering flap that covers the basal portion of the
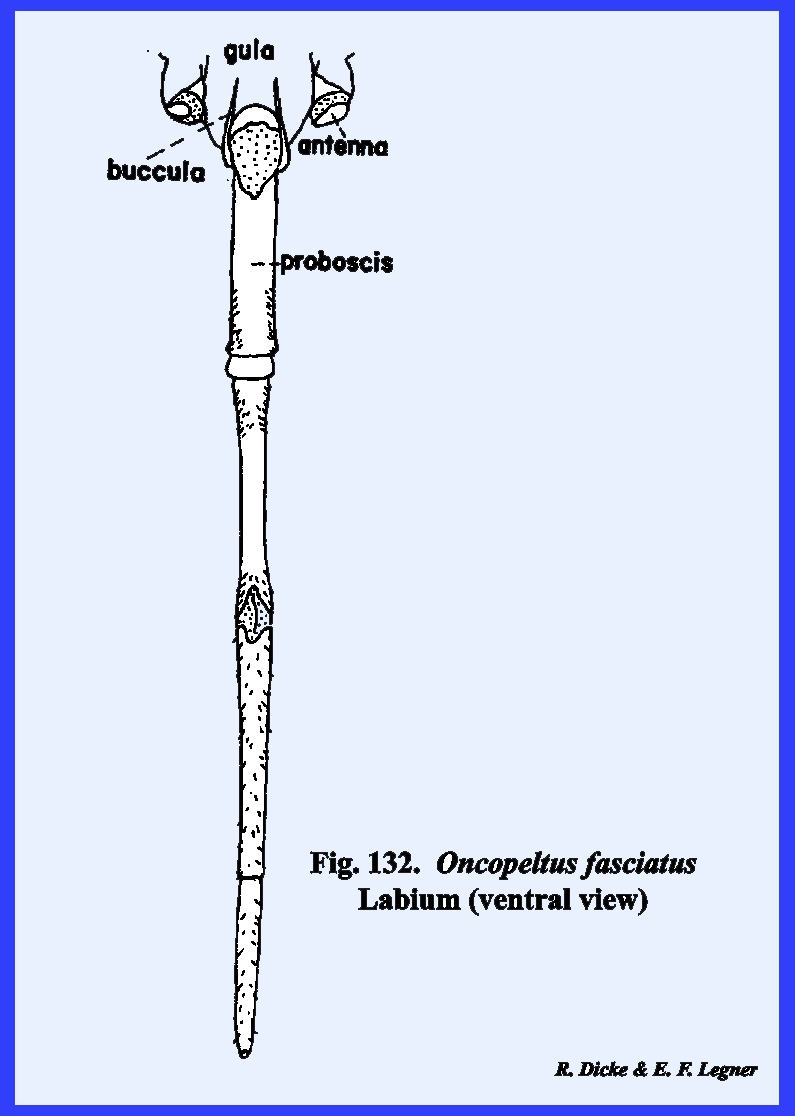
proboscis (Fig 41). The ventral floor of the head comprises a
large sclerite termed the gula, and margined by sutures referred to as gular
sutures. Again, this identification
is uncertain since the gular sutures do not appear to be homologous with a
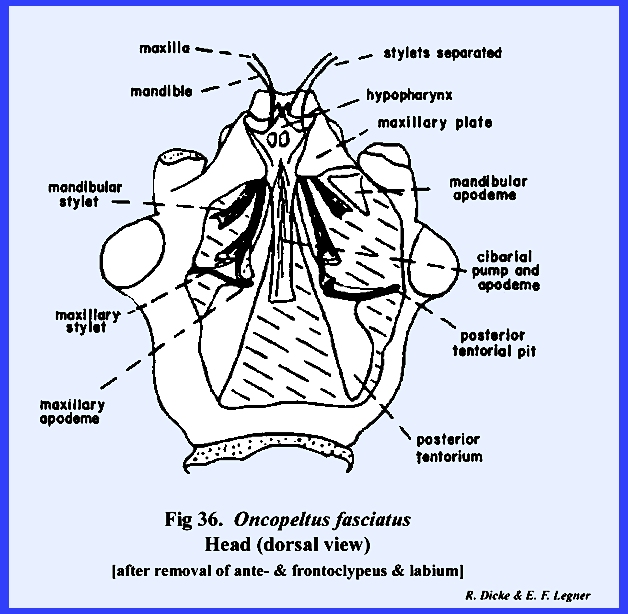
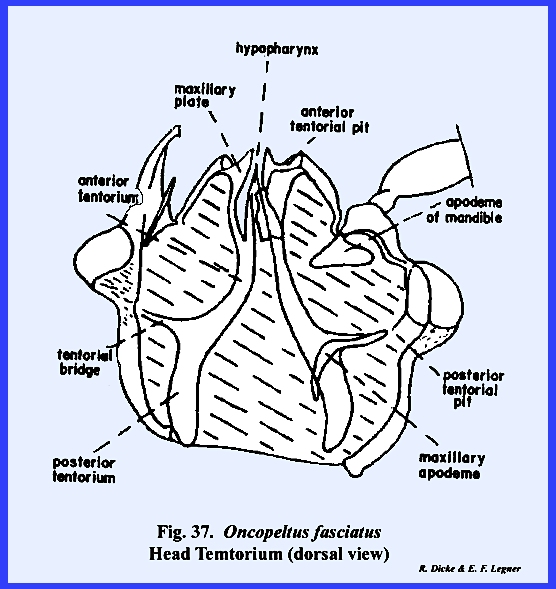
postoccipital suture bearing the posterior tentorial pits. The tentorium is modified into two
arms without a posterior tentorial bridge.
The anterior tentorial pits may be found on the epistomal sulcus, but
the posterior arms are free. Each arm
is anchored posteriorly to the head capsule by lateral projections which are
fixed to the head capsule at a point below the compound eyes, but not on the
gular suture. The proboscis is set in
a membranous area margined by sclerotized ridges. These ridges or elevated plates are referred to as the buccula
(Fig 41)., a descriptive term since their
identification is obscure. The
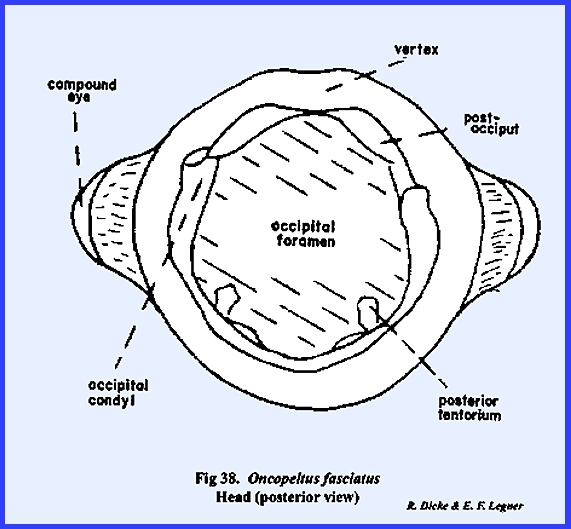
occipital foramen is large (Fig 38), and is
margined by what appears to be a postoccipital sclerite. Since the posterior tentorial bridge is
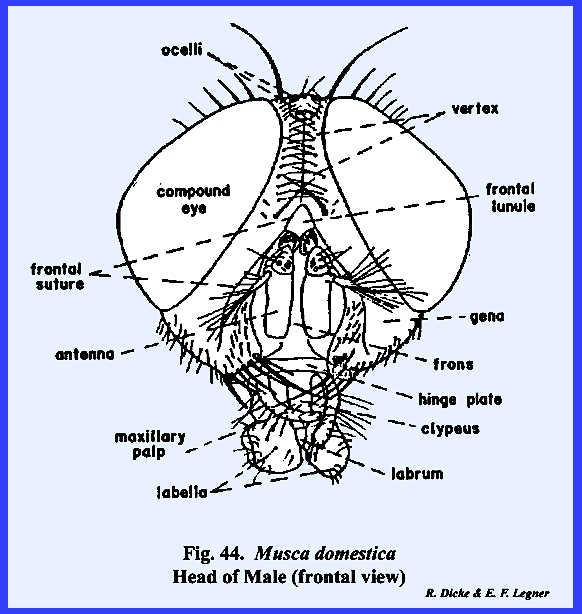
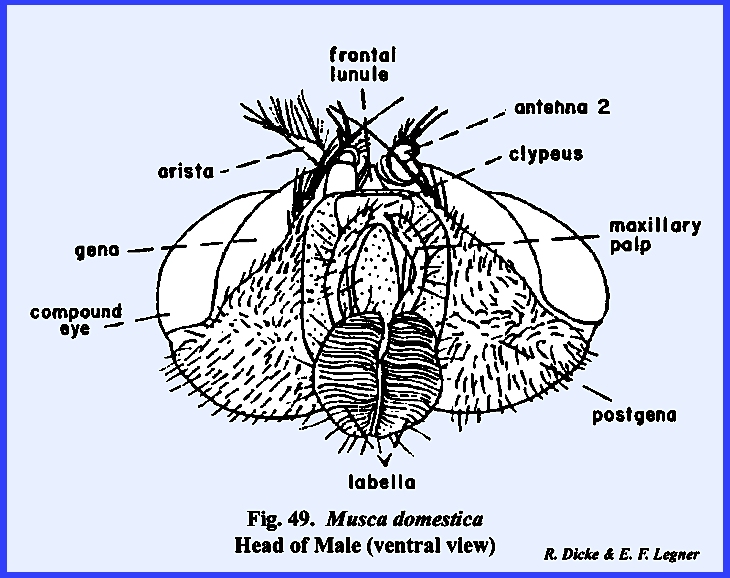
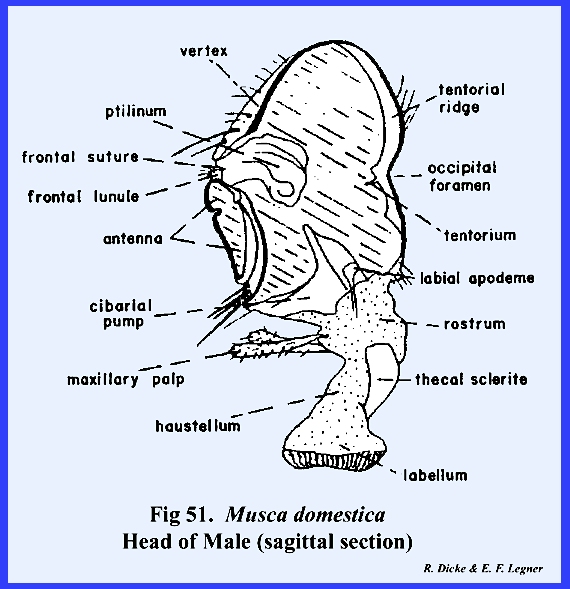
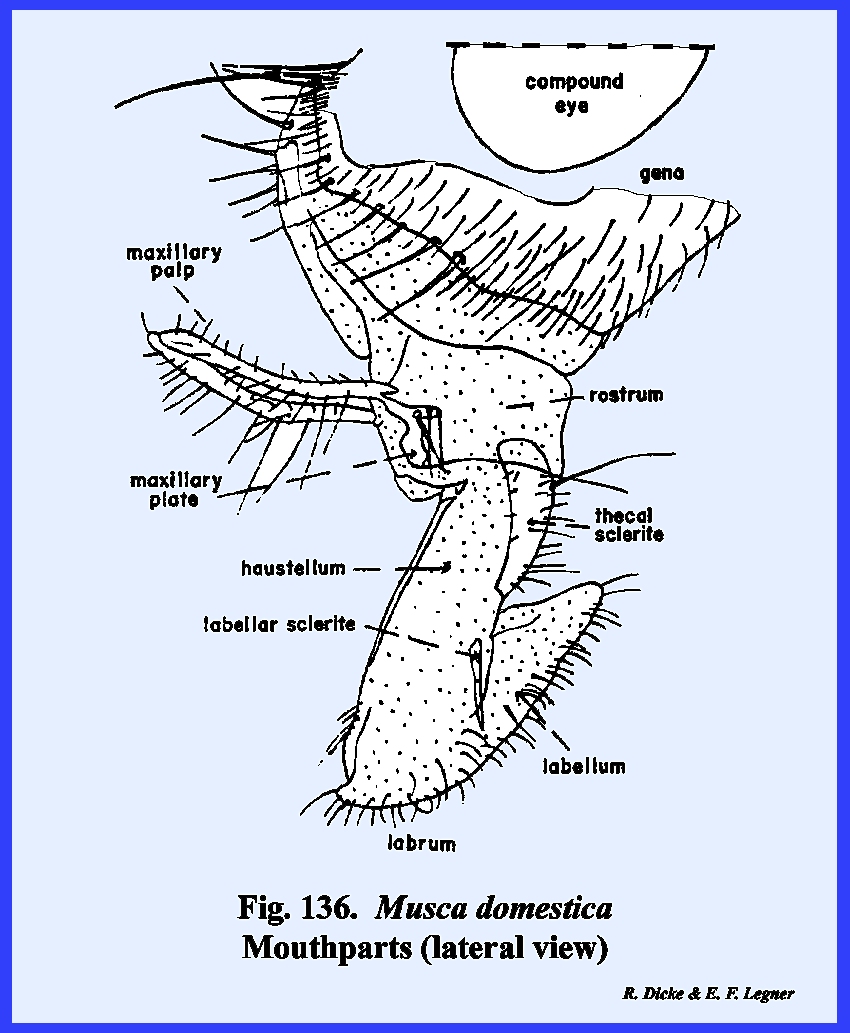
absent, this sclerite is also difficult to homologize. The head of the adult fly is ovoid
and hypognathous with the complicated sucking apparatus pendant in
position. Similar to Oncopeltus fasciatus, the facial areas
of Musca domestica are also
difficult to identify. The tentorium
is greatly reduced and is without anterior arms or a posterior tentorial
bridge. A posterior tentorial ridge has been tentatively identified as a
modified part of the tentorial structure (Fig
51). To further complicate the head structure,
the ptilinum, a peculiar invagination of the head capsule, has
required further modifications of the facial region (Fig 51). The ptilinum is an invaginated sac which
is protruded (along with a distension of the frontal region) bubble‑like
during the emergence of the adult from its pupal case. Although the ptilinum is used only during
emergence, its suture or invagination remains intact. For want of a better term, this suture is
referred to as the frontal suture (Fig 44). However, this
frontal suture of Musca domestica
is not homologous with the anterior arms of the previously described
ecdysial or epicranial suture. The
area enclosed by the frontal sutures is possibly the true frons. An epistomal suture is absent, although a
sclerite termed the hinge plate on the ventral margin of
the frons may represent the epistomal region (Fig 45). A true clypeus, identified by the muscle
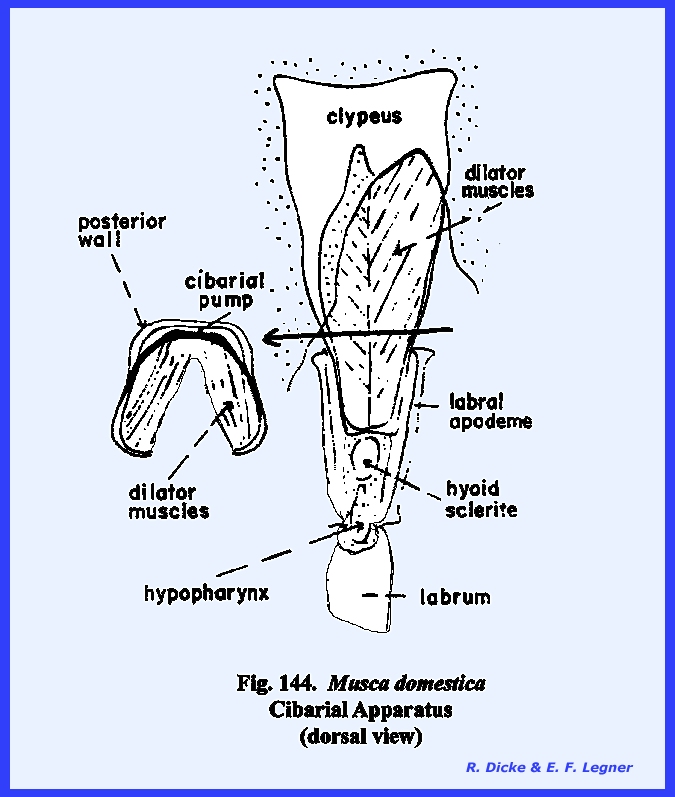
attachments of the pumping mechanism (the cibarlal pump), does occur on the
proboscis and articulates with the hinge plate (Fig 51). Thus far in the description of the facial
area, the clypeus is the only sclerite that can be identified with any degree
of certainty. At the apex of the
frons is a distinct triangular sclerite given the descriptive name of frontal lunule (Fig
45). The marginal
sutures of this sclerite lead directly to the ptilinum. A pair of highly modified antennae lies on
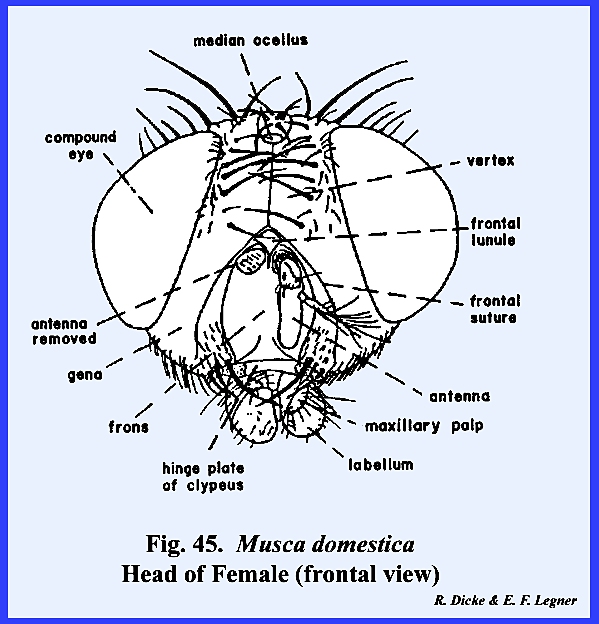
the frons with their base attached to the apex of the frontal lunule. The sclerotized areas between the compound
eyes and frontal sutures are the gena (the "cheeks" of descriptive
entomologists). Dorsad of the frontal
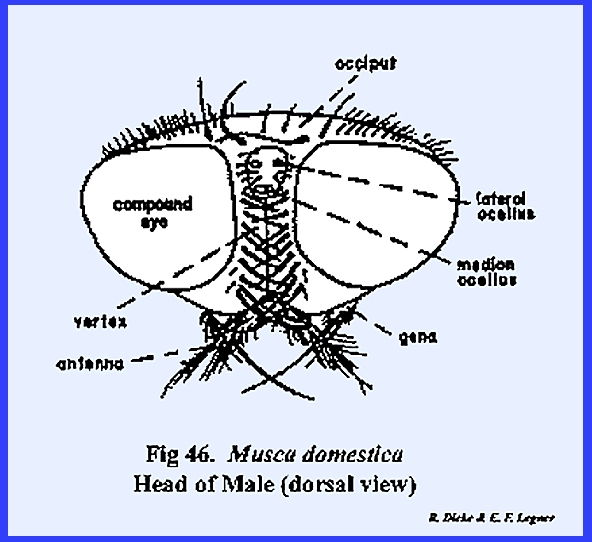
lunule is a sclerotized area identified as the vertex. At the apex of the vertex is a distinct
protuberance or chalaza bearing 3 simple eyes in a cluster. A sexual dimorphism is evident in that the
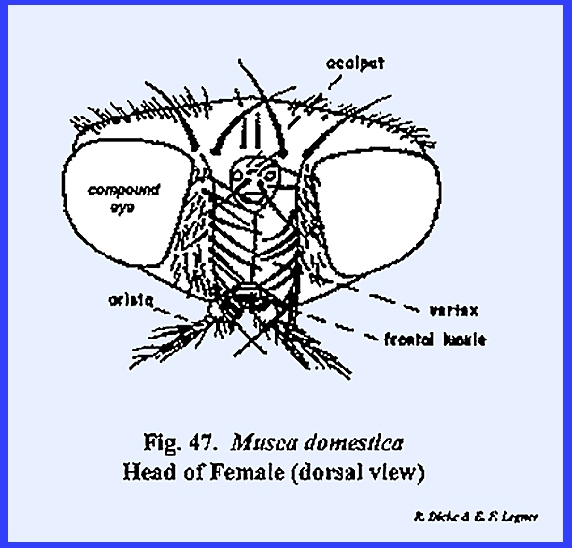
vertex of the male is narrow compared with that of the female (Figs 46 & 47). Since the eyes appear to be set close
together in the male, this condition is referred to as holoptic. In the female, the head is dichoptic, or a condition in which the eyes are set
comparatively wide apart. In the absence of a distinct
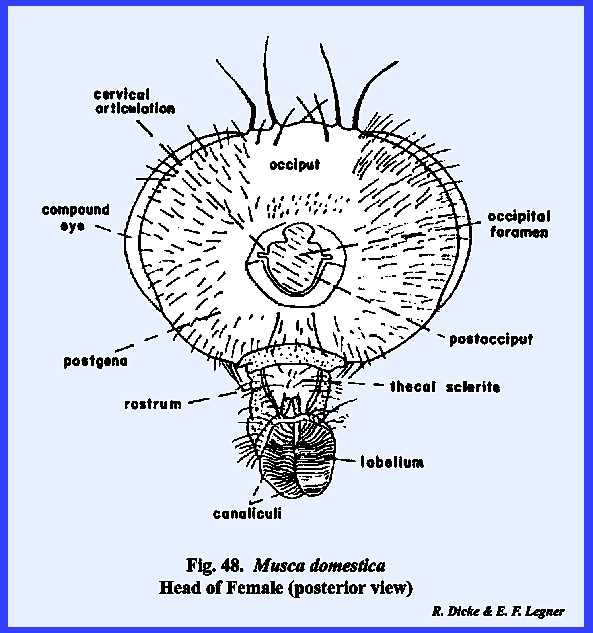
tentorium, the posterior regions are also difficult to homologize. The occipital foramen is comparatively
small. A ridge margins its ventral
aspect, which is probably the postocciput.
An occipital suture is absent, although the dorsal area of the broad
posterior aspect of the head is usually referred to as the occiput. Its lateral and ventral aspects are
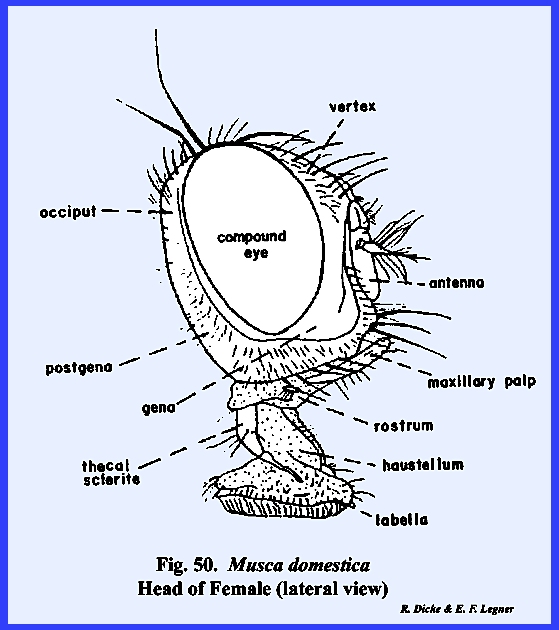
identified as the postgenae (Fig 48). The fleshy proboscis is divided into two
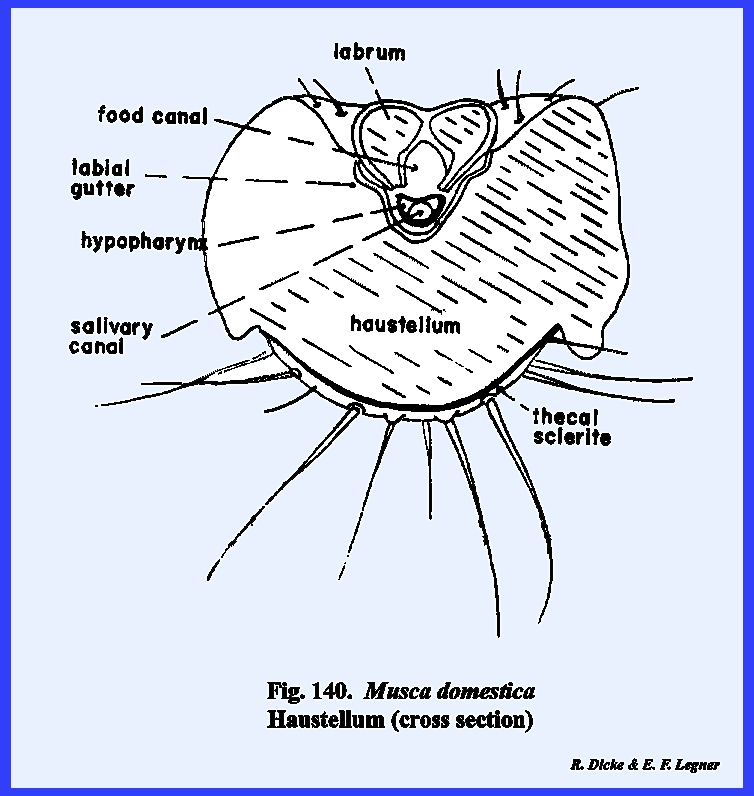
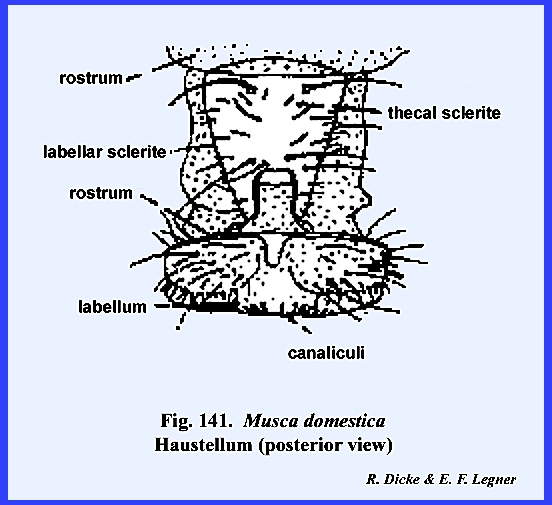
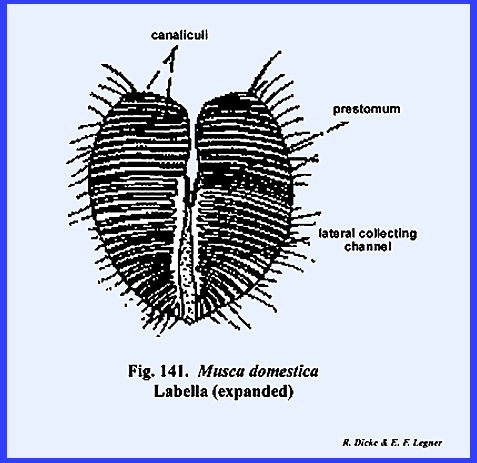
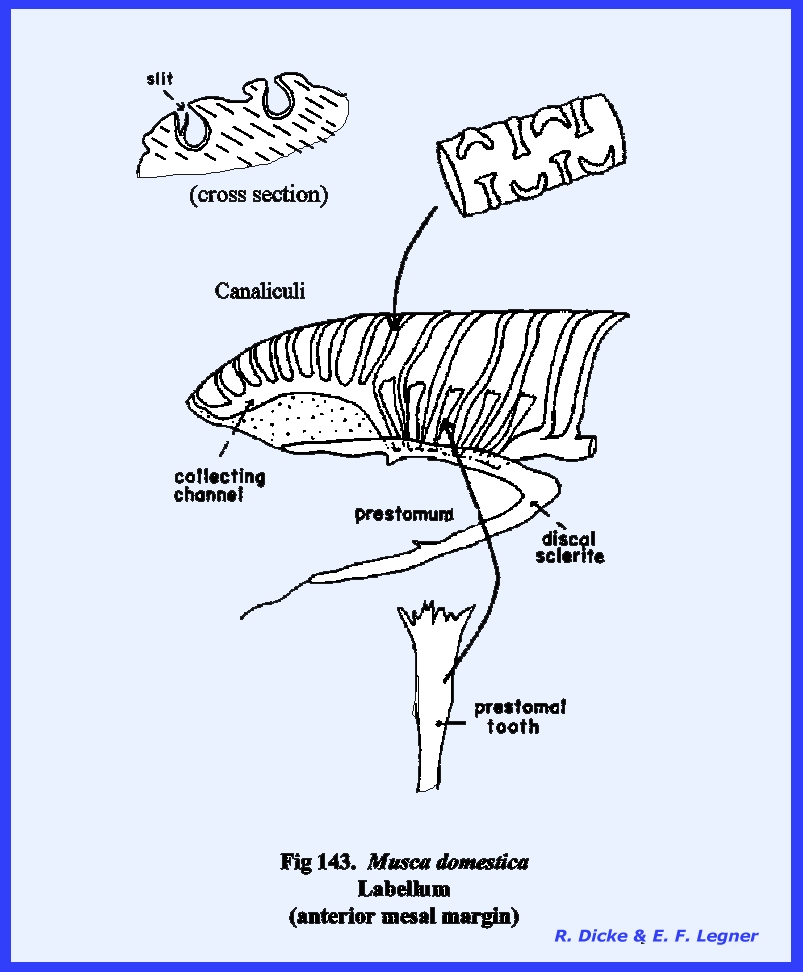
distinct parts referred to as the rostrum and haustellum (Fig 50). This complex structure will be discussed
in detail in the following section III. Specializations
in the Head Capsule of the Immature Insects
The body form of a grub or
caterpillar might suggest that these worm‑like immatures are primitive
in form, but this assumption would be far from accurate. The larvae of the holometabolous insects
are highly evolved forms modified to meet a particular food niche. The head structure ranges in complexity
from the grub of Phyllophaga rugosa
to the maggot of Musca domestica. Little difference would be observed in the
head of an adult or immature Thermobia
domestica, and the nymphal head of Leucophaea
maderae is comparable with the adult.
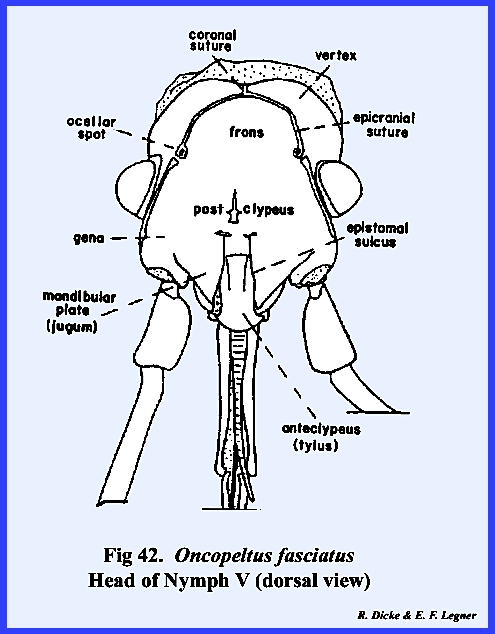
The nymph of Oncopeltus
fasciatus is also comparable with the adult. The presence of a well-developed epicranial suture in the nymph
is the important exception, and the gular region may be incompletely
developed. The epicranial suture in Oncopeltus fasciatus is obviously an
ecdysial suture. It is absent in the
adult of Oncopeltus fasciatus
although it is retained in the adult of Leucophaea
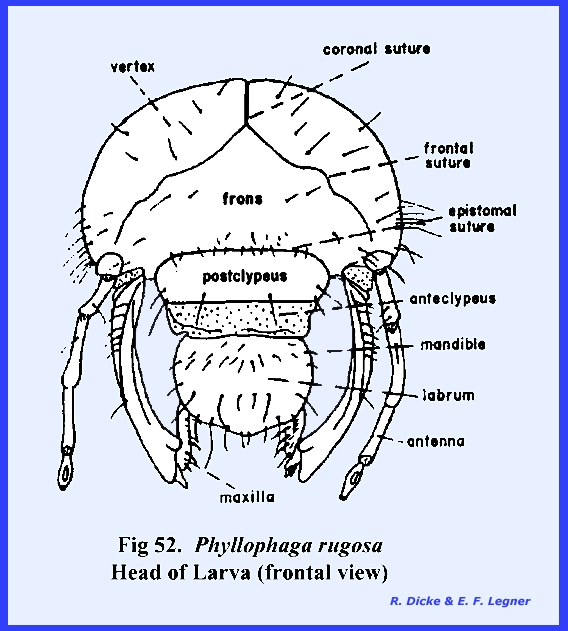
maderae. PHYLLOPHAGA RUGOSA Larva: The facial regions are readily
identified in the grub of Phyllophaga
rugosa. A distinct epicranial
suture is present. Both compound and
simple eyes are absent. The tentorium
forms a strong posterior tentorial bridge, but the anterior arms are weak and
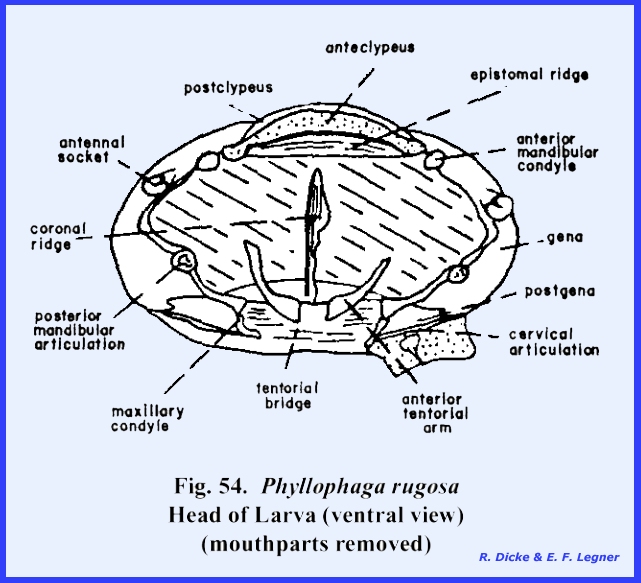
do not extend to the epistomal suture (Fig 52). As a compensation for these weak tentorial
arms, it should be noted that the margins of the mouth cavity are strongly
sclerotized to accommodate the articulation of the chewing mouthparts. The epistomal suture also forms a strong
epistomal ridge (Fig 54) which is an
apodeme bracing the ventral aspect of the head. A distinct labrum is present, and the clypeus is divided into
an anterior membranous anteclypeus and a posterior sclerotized postclypeus. Unlike the adult, no posterio‑ventral
development has occurred, and the chewing mouthparts of the hypognathous head
are pendant as in Leucophaea maderae. The larval head is unique in its
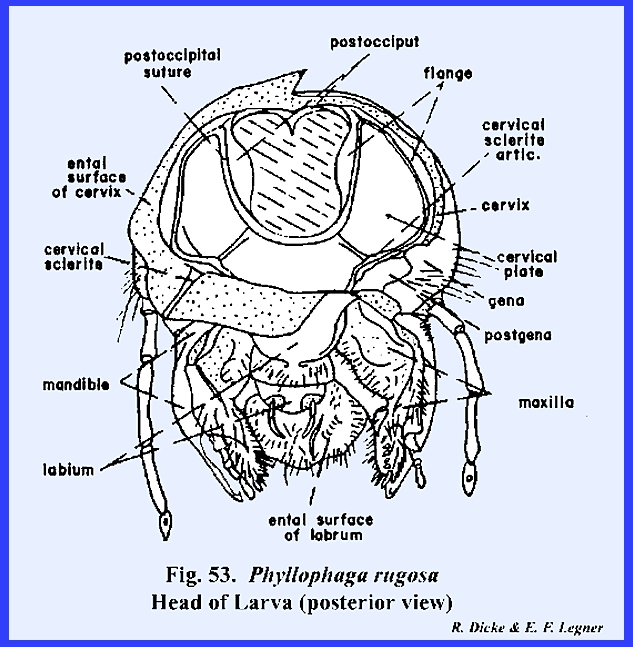
posterior development. A
postoccipital suture is present along with a narrow, laterally flanged
postocciput. Fixed to this sclerite
is a broad plate which is attached to the
posterior aspect of the head. This
plate is identified as a cervical plate since it is a
sclerotization probably derived from the cervix (Fig 53). The membranous cervix proper is joined to
the outer margins of the cervical plate giving the cervix a broad truncate
attachment. Actually, the occipital
foramen is considerably smaller than is indicated by the broad attachment of
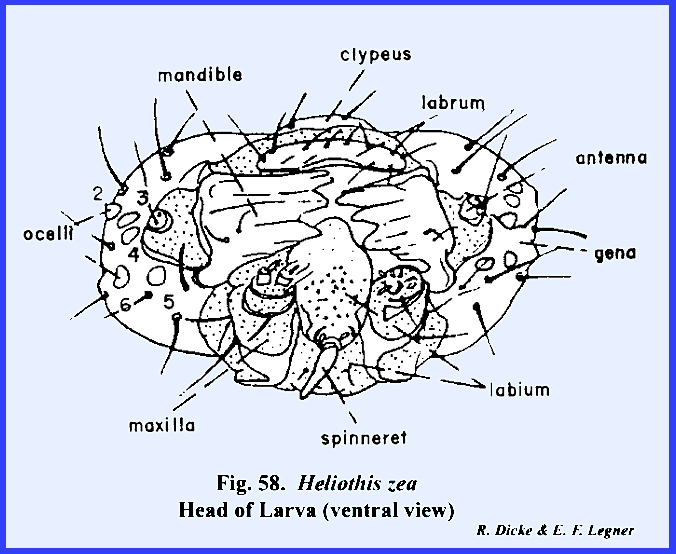
the cervix. HELIOTHIS ZEA Larva: At first, the facial region of the
larval Heliothis zea appears as the
generalized form of an immature insect.
There seems to be a distinct epicranial suture enclosing a triangular
frons. In fact, quite incorrectly,
the head of a caterpillar such as Heliothis
zea has been illustrated as a form with a "typical" epicranial
suture. However, examination of the
tentorium shows that the small anterior arms are attached about midway to the
so‑called frontal sutures. This
condition, then, is similar to the adult of Apis mellifera. The
suture may now be correctly identified as a strongly arched epistomal suture,
and the area enclosed by it is the clypeus (Fig 57). The partially sclerotized area between the
triangular clypeus and the labrum may be correctly identified as the
anteclypeus. An examination of the
endoskeleton reveals that the stem of this suture forms a strong internal
apodeme. It seems, then, that the
incorrectly identified coronal suture is in fact an invagination of the
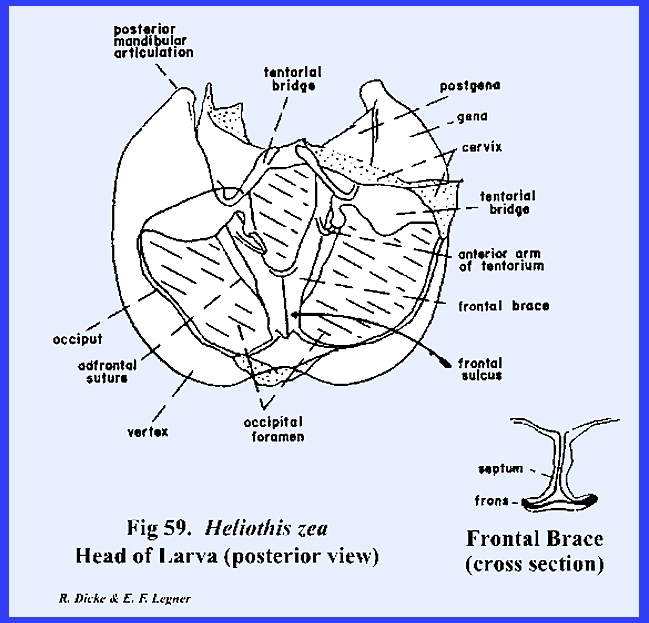
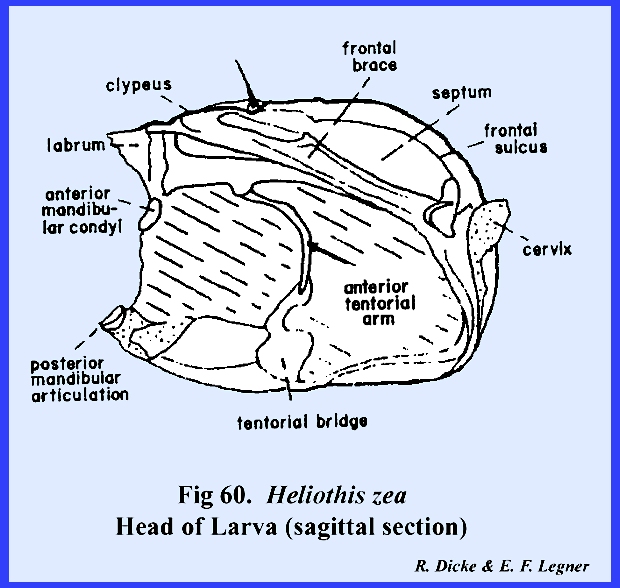
frons to form a strong internal plate‑like ridge. This suture is correctly termed the frontal sulcus (Fig 60). The
tentorium forms a fairly strong posterior tentorial bridge, although the
anterior arms are weak. In the
absence of an effective tentorium, invaginations of the epistomal suture and
frontal sulcus ‑ the frontal brace ‑ provide the
strong endoskeleton necessary for strengthening the head capsule (Fig 60). The combined epistomal suture and frontal
sulcus do serve as an ecdysial suture during molting of the larva. Functionally, then, this combined Y‑shaped
suture could be identified as an epicranial suture. However, anatomically the suture is not homologous with the
epicranial sutures of other immature forms such as Oncopeltus fasciatus, Phyllophaga
rugosa or Apis mellifera. Peculiar to lepidopterous larvae is a
secondary weak suture, which parallels the epistomal suture. It has been suggested that this suture
represents the frontal arms of a primitive ecdysial suture and that the area
enclosed by them is a remnant of the frons.
Since there is little evidence to support this, the suture must be
identified for the present by its descriptive name, the adfrontal suture, and the area enclosed by it as the
adfrontal area (Fig
59). The regions
laterad of the clypeus are the genae and dorsad, the vertex since it was
shown that the frons is invaginated.
Compound eyes are never found in the immature forms of such
holometabolous insects as Phyllophaga
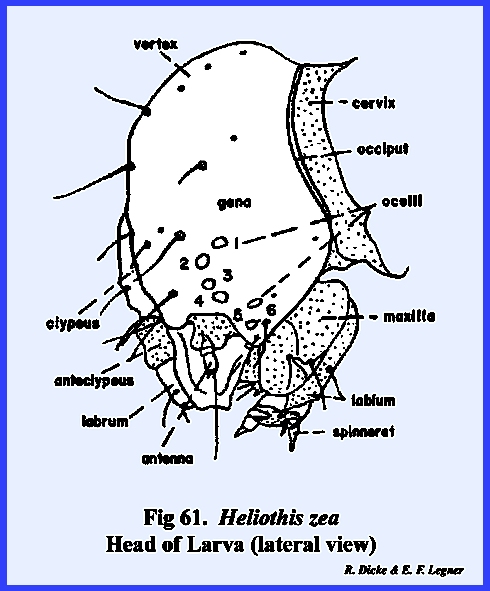
rugosa, Heliothis zea, Apis mellifera and Musca domestica. However, in Heliothis zea six simple eyes (ocelli) occur in a semicircle on
the lateral aspect of the head (Fig
61). Posteriorly,
as in Phyllophaga rugosa, the head
is broadly joined to the cervix.
However, unlike Phyllophaga
rugosa the occipital foramen of Heliothis zea is very broad. An inflexed bridge, which margins the
occipital foramen, may be referred to as either the occiput or postocciput. Modifications in the antennae and
mouthparts as will be described later, also indicate that the hypognathous
head of Heliothis zea is highly
specialized. APIS MELLIFERA Larva: Unlike the free‑living
larvae of Phyllophaga rugosa and Heliothis zea, the grub of Apis mellifera is the ward of a
socialized system and is cared for by the worker bees within a protective
comb cell. It might be assumed that
there would be little need in this larva for the development of strong,
efficient organs of ingestion or effective organs of sensory perception. Actually, the head of Apis mellifera has evolved in the direction of simplification. The mouthparts with the exception of a
silk organ, are greatly reduced, and the organs of sensory perception are
reduced to functionless vestiges. A
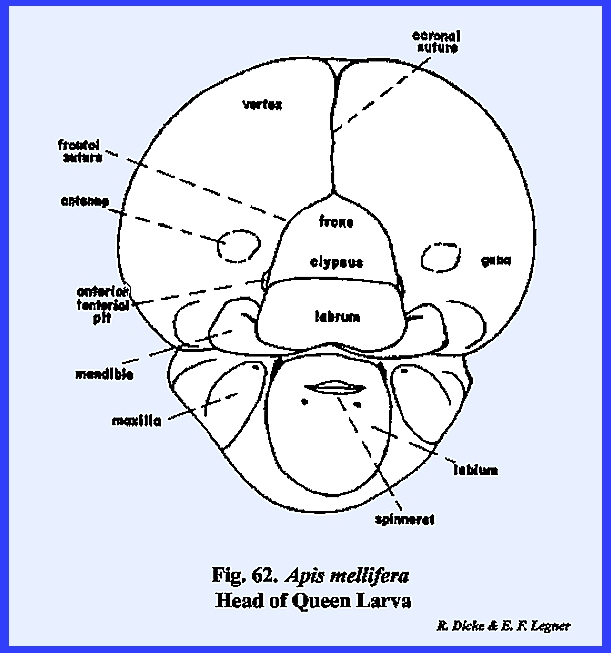
well-developed epicranial suture encloses a fronto‑clypeal region (Fig
62). However,
identification of this region is somewhat uncertain, because an epistomal
suture is absent, and the anterior tentorial pits appear at the distal ends
of the frontal sutures. But, an
examination of the endoskeleton gives little reason to assume that the arms
of the Y‑shaped suture are morphologically comparable to the condition
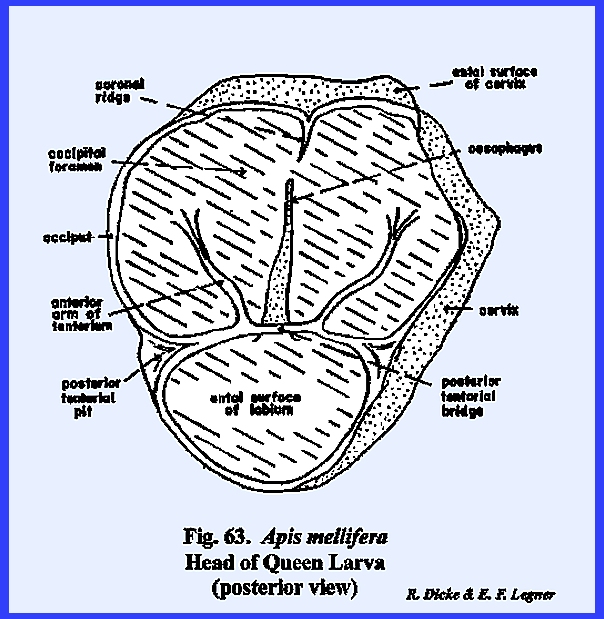
described for Heliothis zea. The tentorium is a typical TT‑shaped
structure with distinct anterior and posterior tentorial pits. Both the posterior tentorial bridge and
anterior arms are weakly sclerotized.
The occipital foramen is very wide, and the head is broadly joined to
the thorax without a distinct cervix.
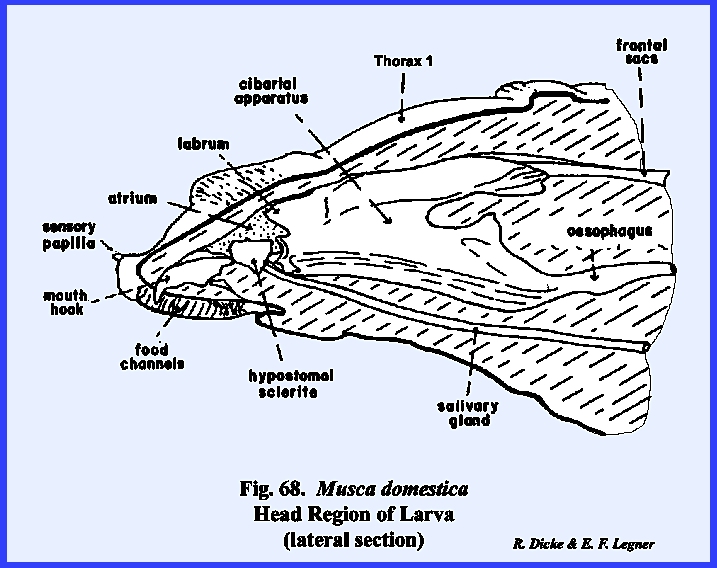
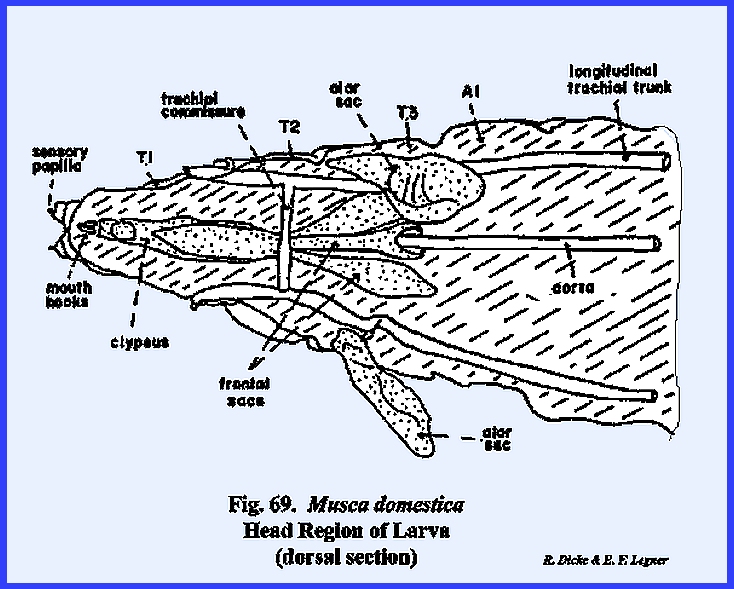
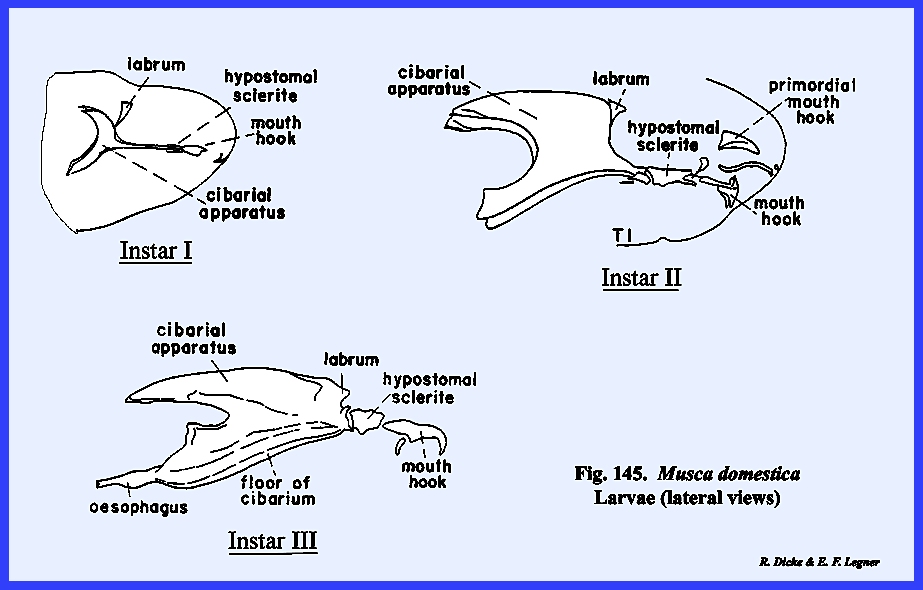
MUSCA DOMESTICA Larva: The larva of Musca domestica is a very active, free‑living form. But unlike Phyllophaga rugosa and Heliothis
zea, the head has been greatly modified and reduced drastically from the
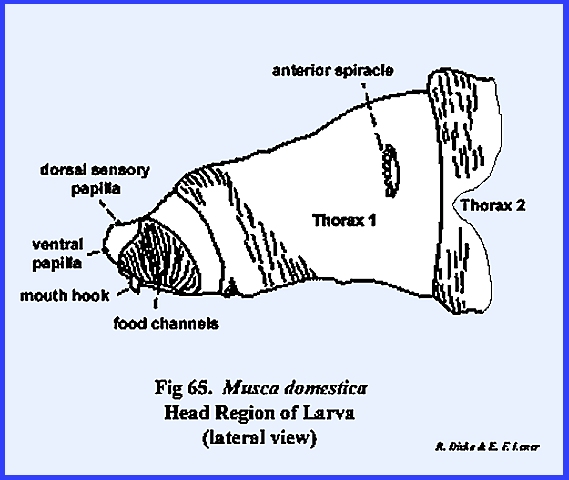
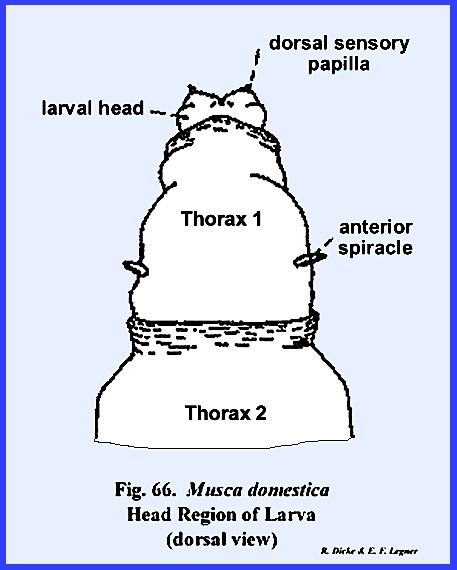
typical form (Fig 65). A conspicuous anterior segment, which may
be readily confused as a cylindrical head, is actually the first thoracic
metamere. This metamere may be
identified by a pair of respiratory structures (anterior spiracles) which are
never known to occur on the head region.
The small lobe anterior to this metamere represents the head of the
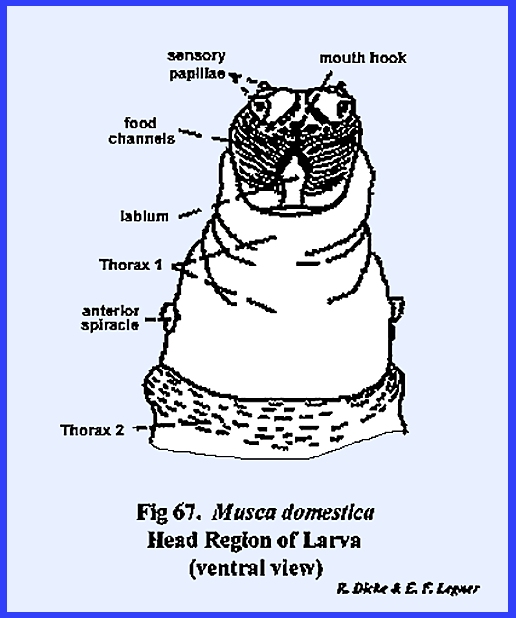
maggot since there is a mouth opening on its ventral aspect. A series of grooves lead to and occur on
either side of the mouth opening.
These grooves are the so‑called food channels, and may have the function of
conducting liquids to the mouth opening (Figs 67 & 68). Two pairs of small projections or papillae
occur on the dorso‑anterior aspect of the head. These are identified as the dorsal sensory papillae and
the ventral sensory papillae. The papillae are
apparently sensory in function, but are not in any way homologous with
antennae or eyes. Protruding from the
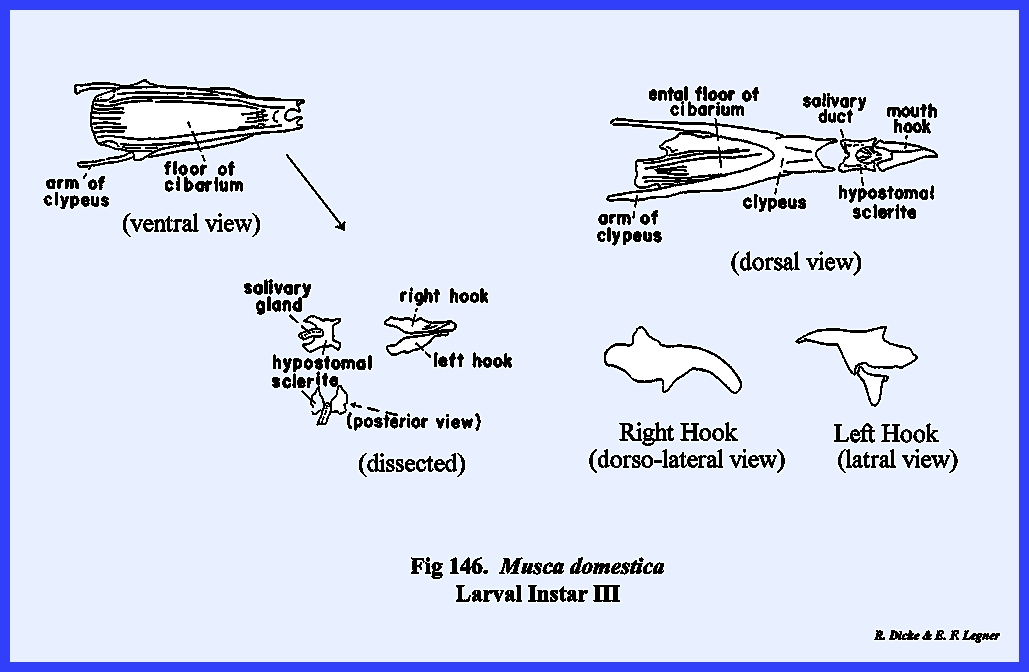
mouth opening is a hook‑like structure identified as a mouth hook used for procuring and ingesting food. Dissection of the larva reveals structures
such as the sucking pump (cibarlal apparatus), which are homologous with
similar structures in the adult (Fig
68). These will
be discussed in detail in the next section III. For the present, it is apparent that the mouthparts and certain
sclerites of the head are deeply invaginated within the body cavity. It is also apparent that all of the head
capsule but the food ingesting apparatus has been retarded in
development. The primordial cells for
the adult head including the sensory structures are found within internal
sacs known as the frontal sacs (Fig
69). These primordial sacs are retracted deep
in the body cavity. Careful
dissection shows that the anterior channels of these sacs actually open into
the mouth cavity. During the pupal stage,
the primordial cells within the sacs grow very rapidly and to the extent that
the sacs are evaginated to the exterior.
The head capsule and frontal region of the adult are then placed in an
external, anterior position. It then
becomes apparent that the functional head of the maggot is not comparable
with a typical head capsule, and that such structures as the mouth hooks and
sensory papillae are secondary but highly evolved organs. SECTION III - THE MOUTH PARTS
The organs developed for the
ingestion of food collectively referred to as mouthparts, may for most
insects be functionally classified as either mandibulate
or haustellate. Mandibulate mouthparts probably occurred
early in the evolution of insects and for the most part were primary modifications
of existing appendages remodeled through the process of selection for
grasping, biting and chewing solid foods.
Haustellate mouthparts probably were further elaborations of the
mandibulate types for the purpose or rasping or piercing and for sucking
liquid foods. While mandibulate
mouthparts usually occur in such primitive forms as Thermobia domestica or Leucophaea
maderae, they may be retained in part by such highly evolved forms as Apis mellifera or by the larvae of Heliothis zea. Mandibulate
Mouthparts
The true mouth of an insect is the
anterior opening of the gut track and is represented in the hypothetical
prototype as the oral opening, situated ventrally
between the prostomium and the 2nd metamere.
It was suggested that the ambulatory appendages of the 3rd, 4th and
5th metameres of the evolved head (collectively, the gnaphocephalon) became
associated with the mouth as organs of ingestion. Actually the segments of these mouthparts although highly
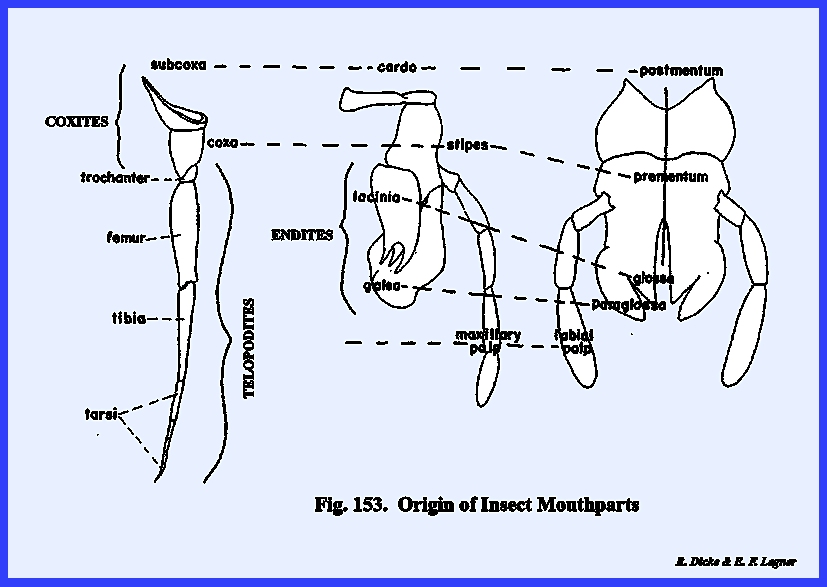
modified can be identified with legs./1 The appendages of the 3rd metamere probably evolved into a pair
of mandibles which serve as a cutting and grinding
mechanism. Appendages of the 4th
metamere evolved into a pair of digitate structures referred to as the maxillae.
And finally, the fused appendages of the 5th metamere or labium
evolved into a plate‑like structure underlying the mandibles and
maxillae. A cranial sclerite, the
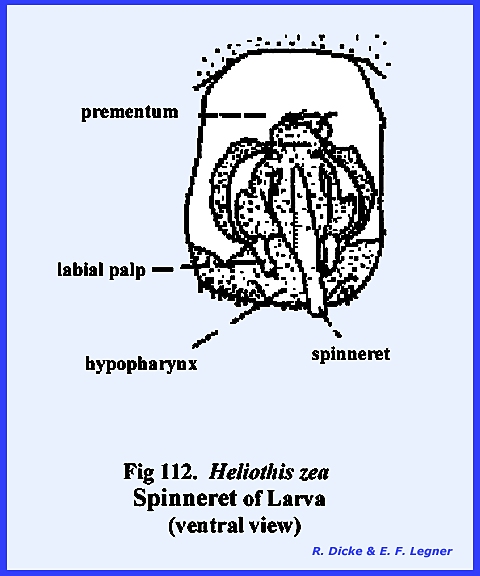
labrum, serves as an upper lip, and a lobe of the head, the hypopharynx serves as a median tongue‑like
structure. All of these mouthparts precede
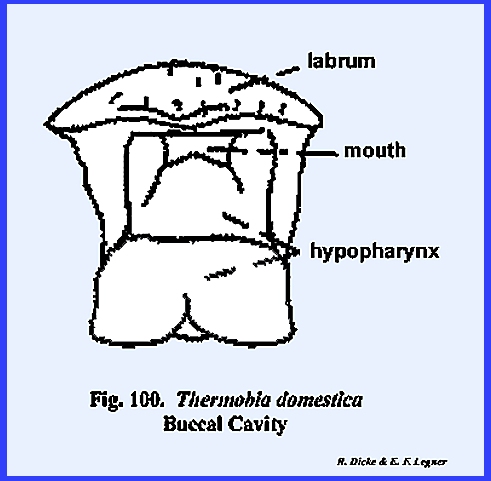
and enclose the true mouth, forming an ingestion cavity identified as the preoral cavity.
The preoral cavity is best visualized as box‑like in formation
with the top covered by the labrum and the bottom enclosed by the fused
labium. Because the mandibles and
maxillae unlike our own jaws articulate on a horizontal plane, these appendages
enclose the sides of the cavity and regulate the opening and closing of the
anterior aspect. The posterior aspect
of the cavity bears the true mouth or opening into the gut and the base of
the median tongue‑like hypopharynx.
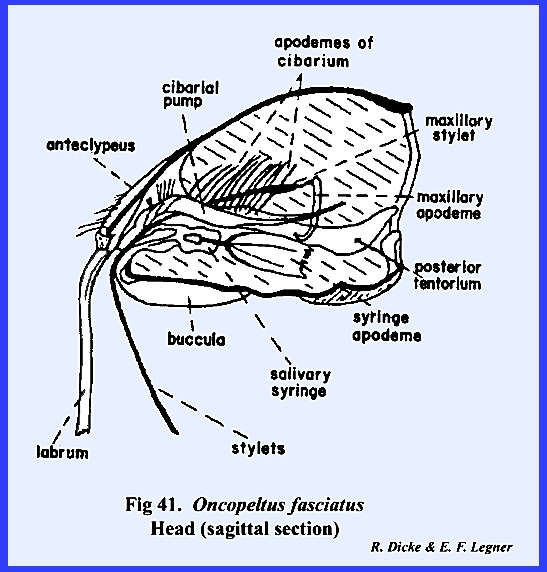
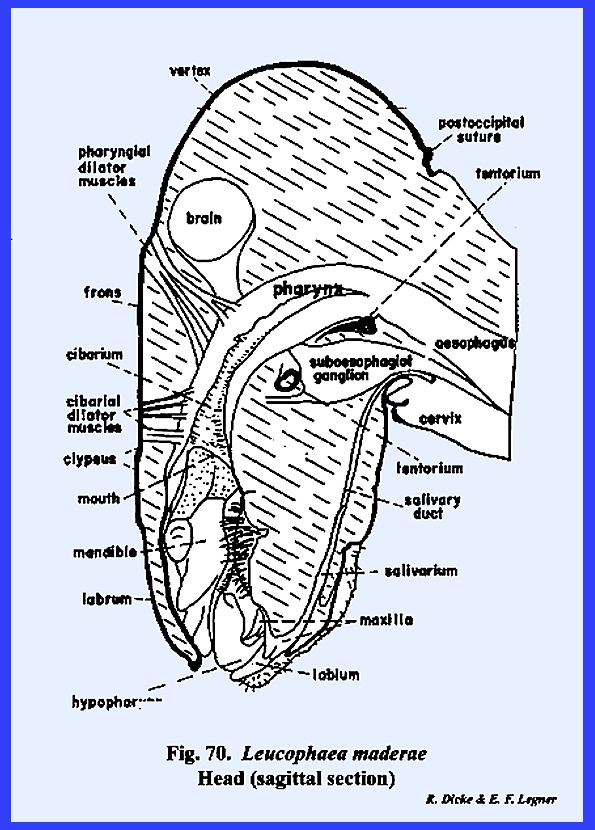
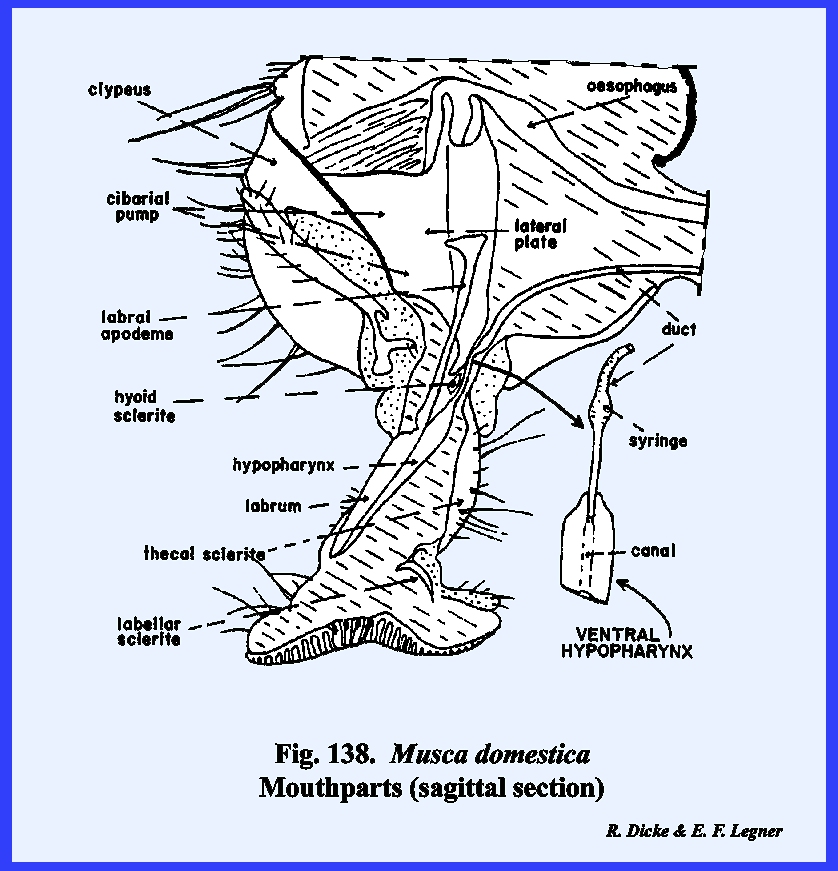
A sagittal section of the head as in Leucophaea maderae illustrates this relationship (Fig 70). Certain areas of the preoral
cavity are identified further. The
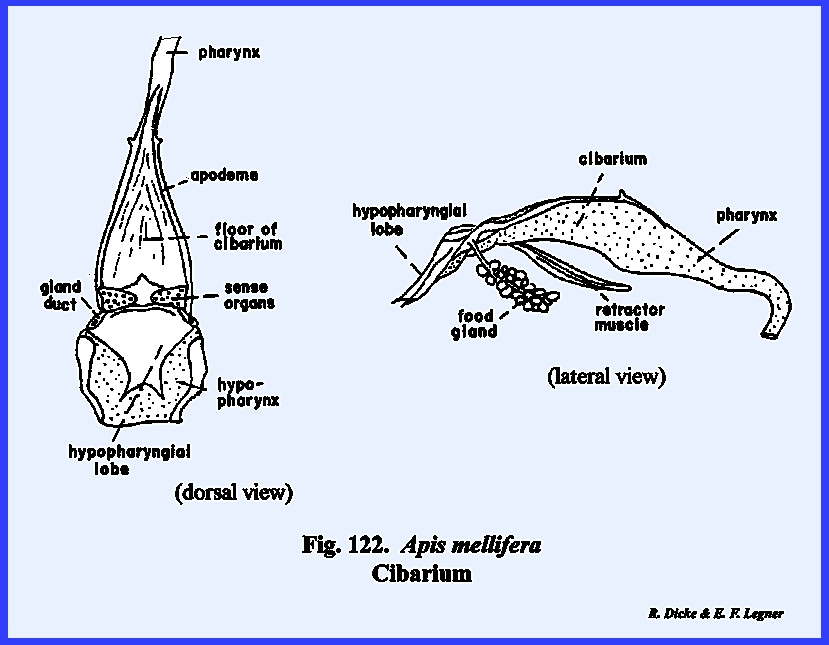
cavity lying directly below the clypeus and above the base of the hypopharynx
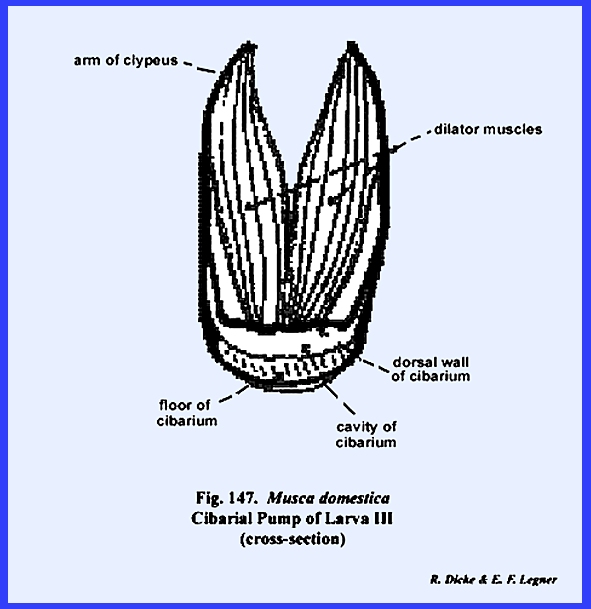
is the cibarium. It may be observed in the sagittal section of Leucophaea maderae that strong cibarial dilator muscles operate
between the dorsal wall of the cibarium and the clypeus. These muscles probably serve an important
function in assisting mandibulate insects to swallow food. Of considerably greater importance is
their specialization into a sucking or cibarial pump in the haustellate
species such as Apis mellifera, Heliothis zea, Oncopeltus fasciatus and Musca
domestica. The cavity formed by
the ventral surface of the hypopharynx and the ental surface of the labium is
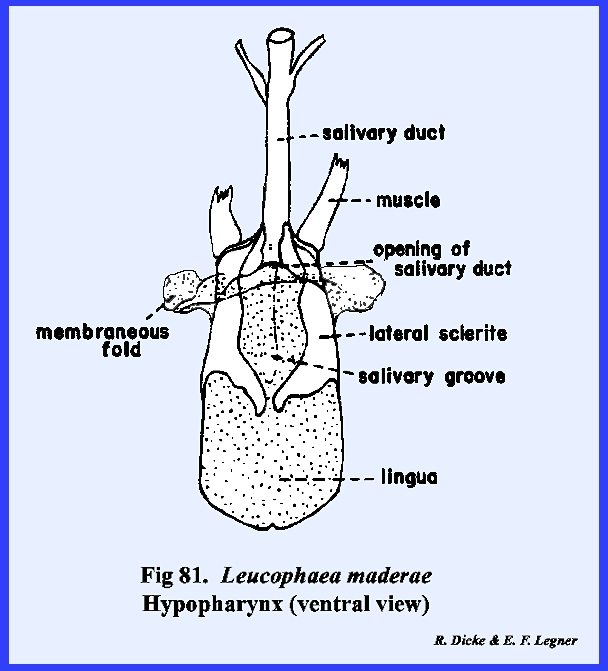
identified as the salivarium. In many mandibulate forms such as Leucophaea maderae, the duct of the salivary gland is situated at the posterior end of this
cavity. ------------------------------------------------ 1/
Refer to Section VI ‑ Origin
of the Mouthparts. The mandibulate mouthparts of Leucophaea maderae provide a good
example of the generalized biting and
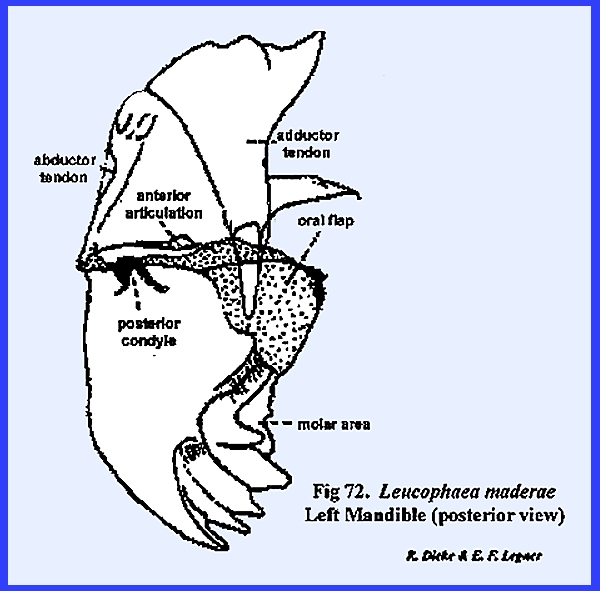
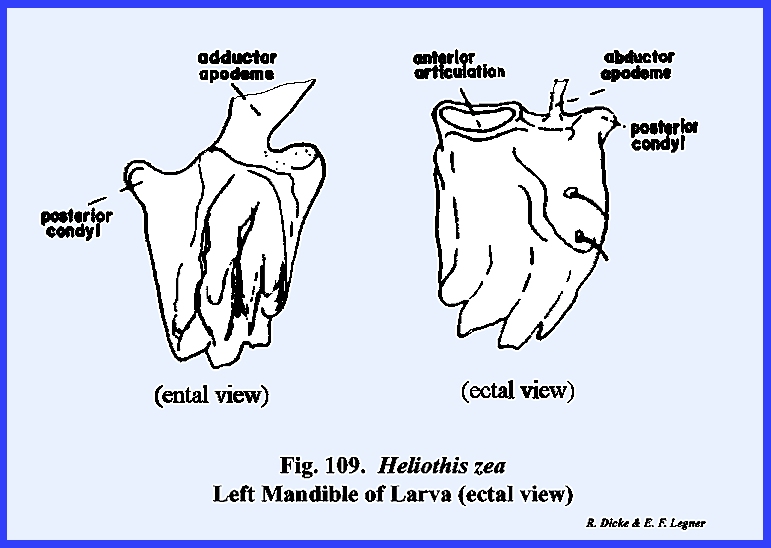
chewing mechanism (Figs 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 & 82). The mandibles are the true jaws designed
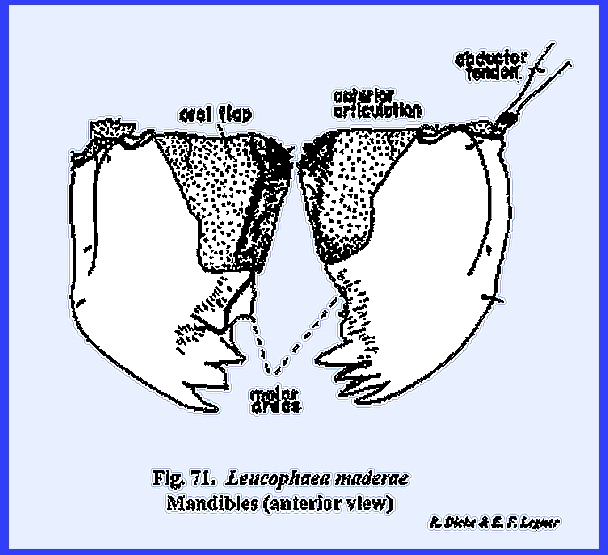
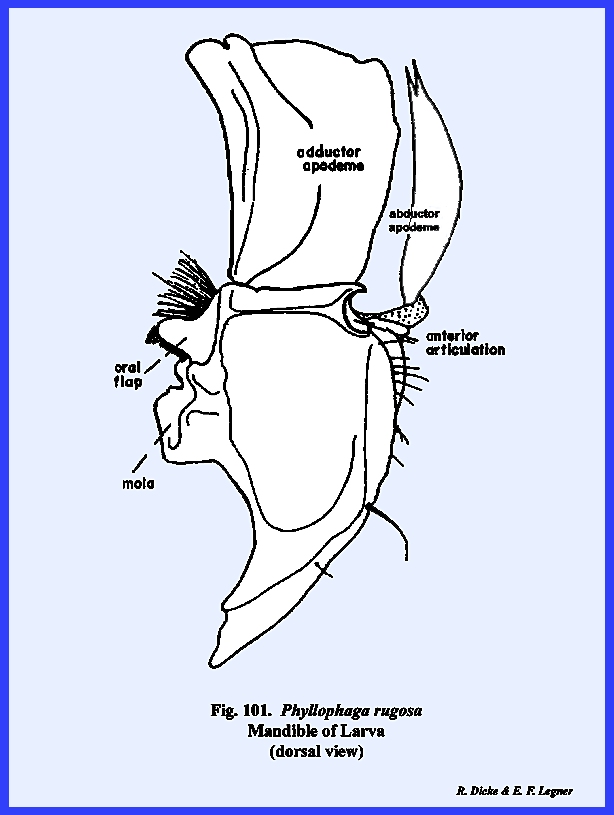
for cutting, tearing and grinding solid foods. In composition they are hollow, unsegmented and usually heavily
sclerotized. The tips of the
mandibles are toothed, and about midway the mesal edge is flattened into a
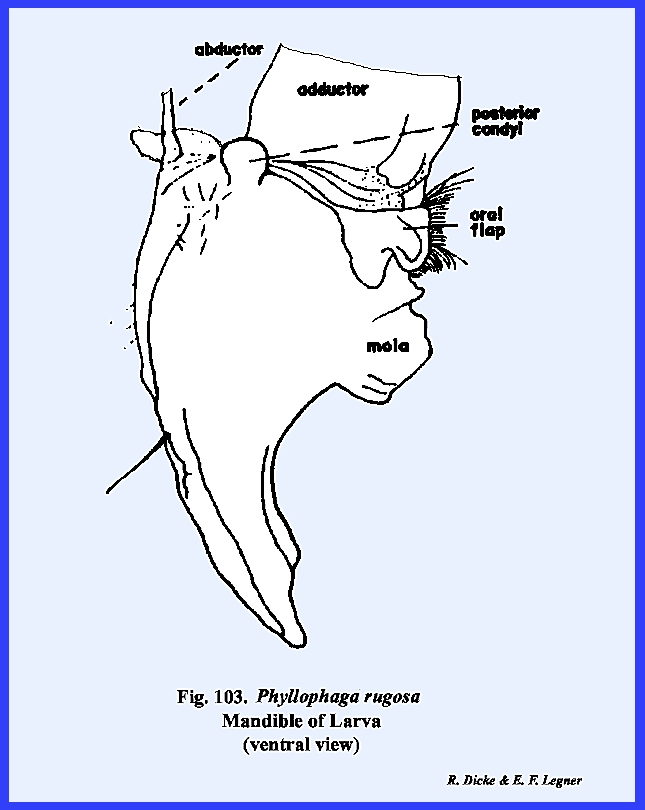
grinding surface designated as the molar area or
mola
(Figs 71, 72 & \). These
mastication areas of the mandible are asymmetrical so that the distal teeth
and the mola will effectively work against each other for cutting and
grinding. In Leucophaea maderae, the basal portion of the mandible is
modified into a soft, resilient lobe or oral flap. The oral flaps seem to have an important
part in the process of swallowing by forcing food particles into the cibarium
as the mandibles are closed together.
The masses of setae on the mesal edges probably serve to hold the food
particle as it is being forced backward.
Outside of the oral flap, few setae occur on the mandible in Leucophaea maderae. In other species such as Phyllophaga
rugosa setae may be distributed profusely over the mandibular surface (Fig 83). Each mandible is attached to and
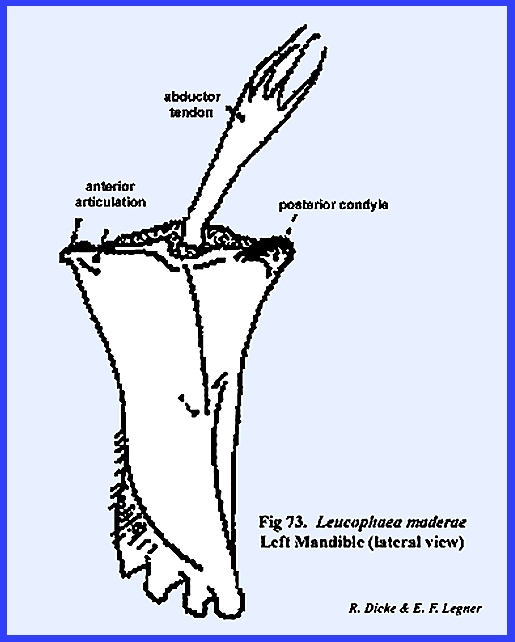
articulates with the head capsule at two points. This dual attachment is referred to as a dicondylic articulation. All other appendages are attached to the
metamere of their origin at only one point or by means of a dicondylic
articulation. Apparently, the
articulation of the primitive mandible was monocondylic,
and in fact this condition does exist in some of the more primitive
Thysanura. The powerful jaws of Leucophaea maderae and Phyllophaga rugosa require a
dicondylic articulation so that the mandibles may be rocked horizontally and
can accomplish a strong mesal thrust.
While the monocondylic mandibles of the primitive Thysanura are
comparatively weak, they appear to be sufficiently effective, however, to
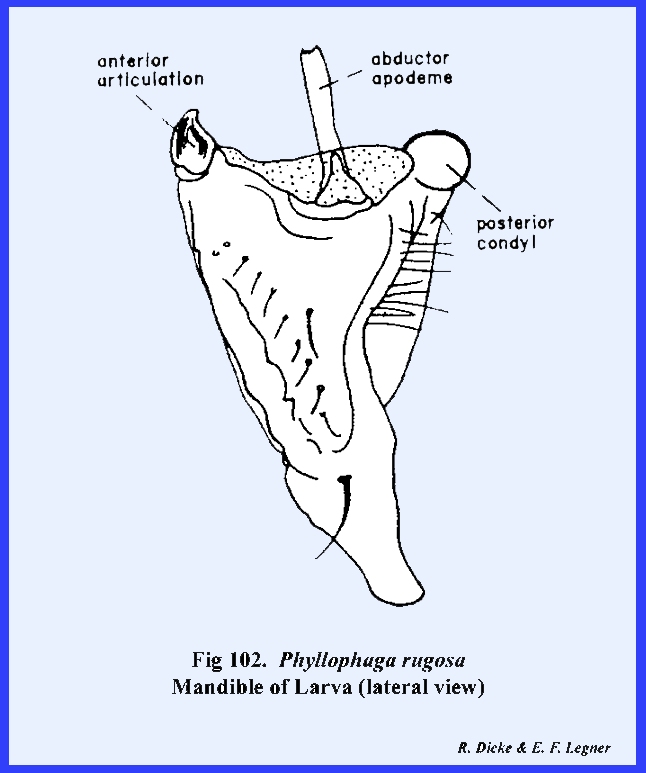
maintain these ancient and quite successful forms. In Leucophaea maderae, the primary (or primitive) point of
articulation is accomplished by means of a knob situated on the posterior
angle of the mandible. This is the posterior condyle which fits into a pocket provided
by the ventral margin of the postgena (Fig 73). The anterior
articulation of the mandible is a much less prominent projection,
which is accommodated by a notch in the lateral margins of the
postclypeus. Two apodemes accommodate
the movement of the mandible. The
adductor tendon is a broad apodeme connecting the
mesal margin of the mandible with a set of powerful muscles. These adductor
muscles close the jaws in the cutting or grinding function. Opening the jaws is accomplished by a
comparatively weak set of abductor muscles attached
to the abductor tendon which operates on the outer
angle of the mandible. The mandibles,
then, are rocked forward with a powerful stroke and backward on a horizontal
plane by two opposing sets of muscles, while the two points of articulation
serve as a hinge. The second pair of mandibulate
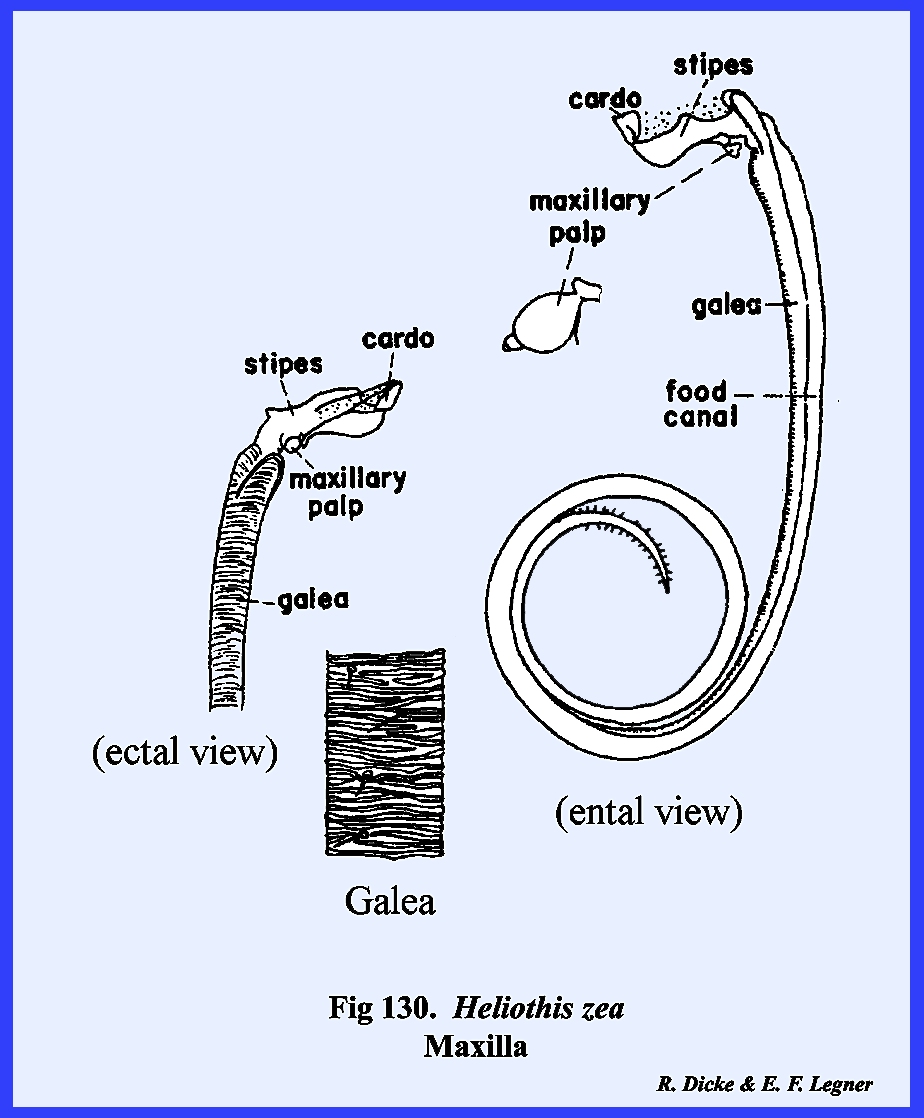
appendages are the maxillae. These
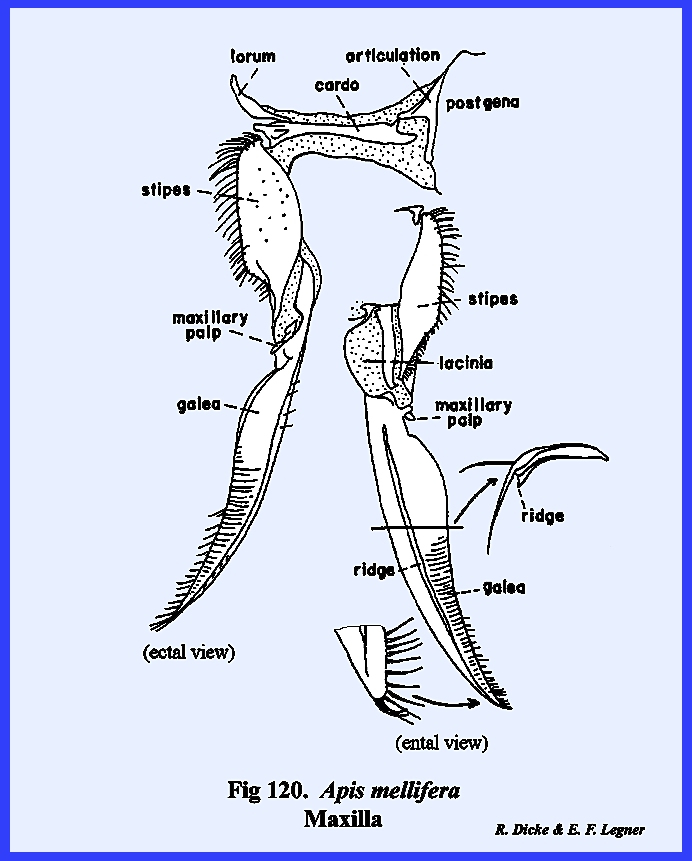
can be reasonably well homologized and most nearly resemble a typical leg. The maxilla is broadly united and
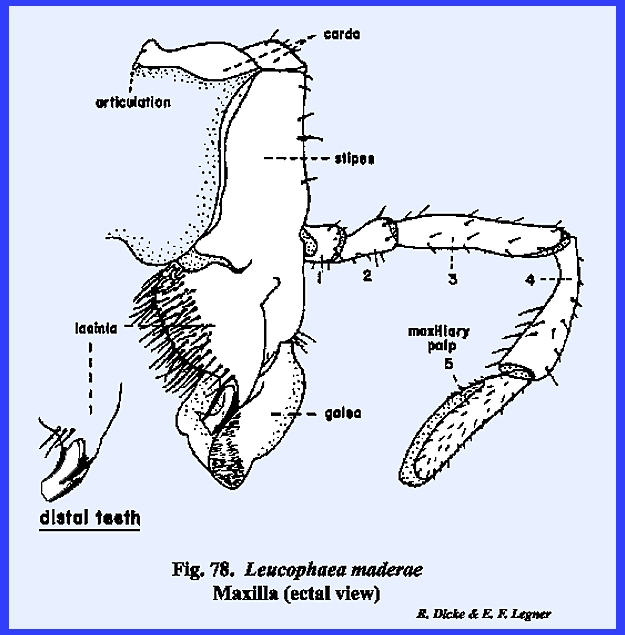
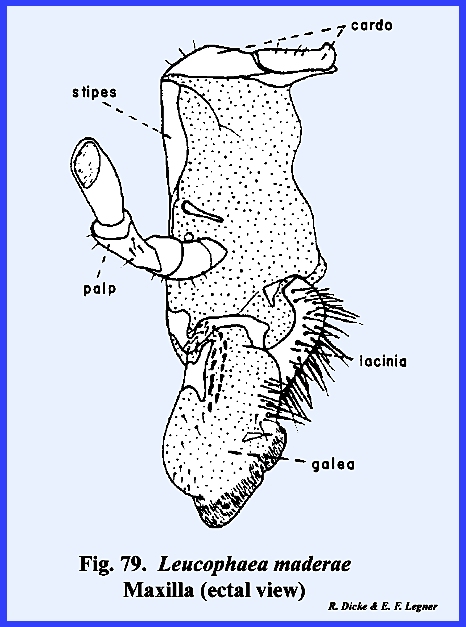
articulates with the ventral margin of the postgena. In Leucophaea
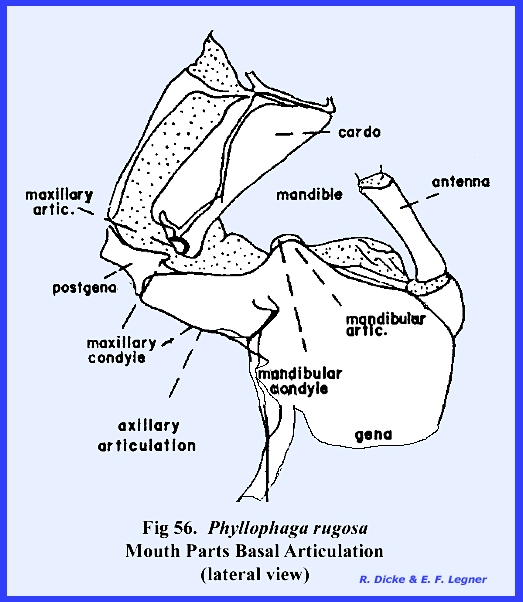
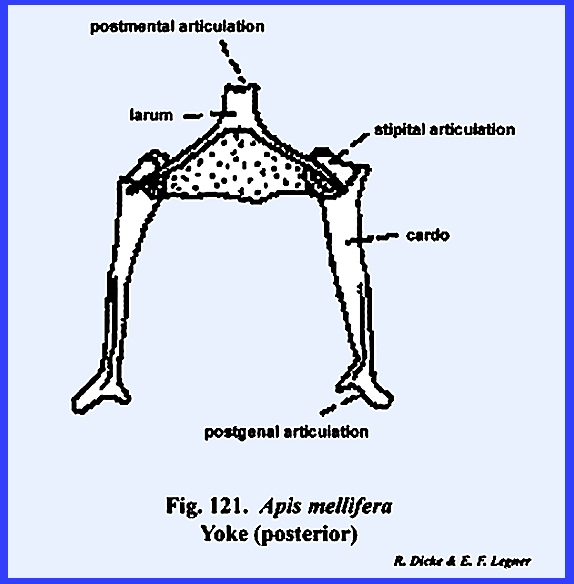
maderae this hinge‑like articulation, the cardo, is 2‑segmented,
and its proximal extremity fits into a notch or maxillary articulation in
the posterior margin of the postgena (Figs 78 & 79). The base of the
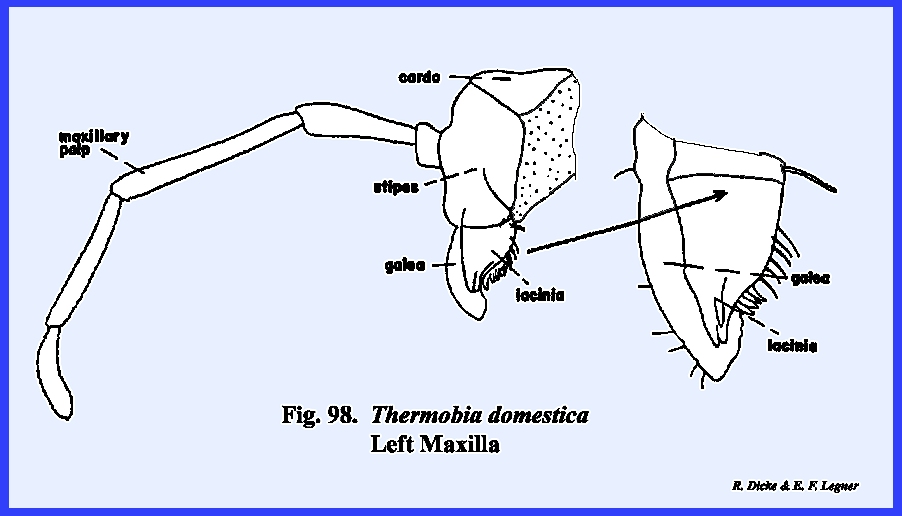
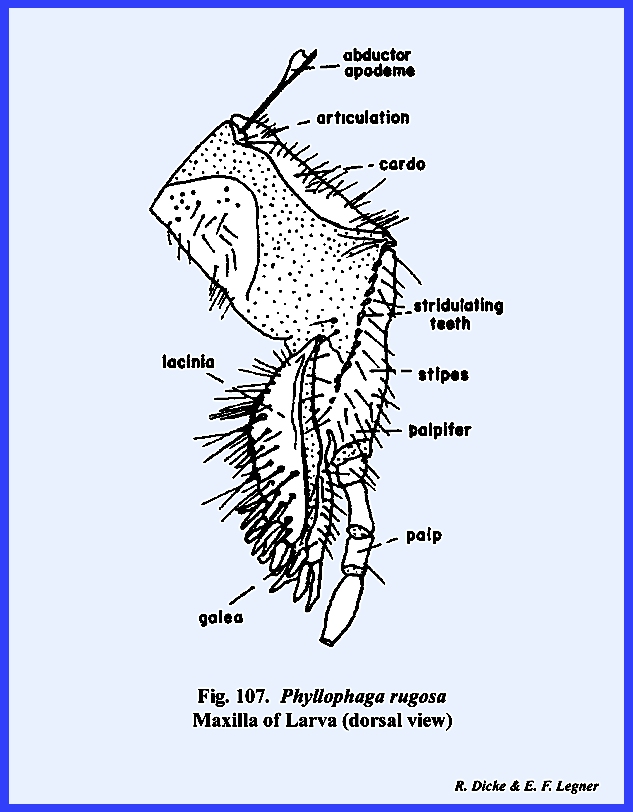
maxilla, or stipes bears laterally a 5‑segmented
palpus and distally two prominent lobes.
The ectal surface of the stipes is heavily
sclerotized and the ental surface is membranous. The palpus, designated as the maxillary
palp, is a finger‑like structure with two short basal segments,
and three long distal segments. The
distal portion of segment 5 is membranous and is probably sensory in
function. In Leucophaea maderae, the maxillary palp articulates directly with
the stipes. Where a distinct sclerite
occurs for articulation as in the adult and larva of Phyllophaga rugosa, this articulatory sclerite is referred to as
the palpifer. The outer
lobe of the stipes is the galea. It is weakly sclerotized except at the base on its ental
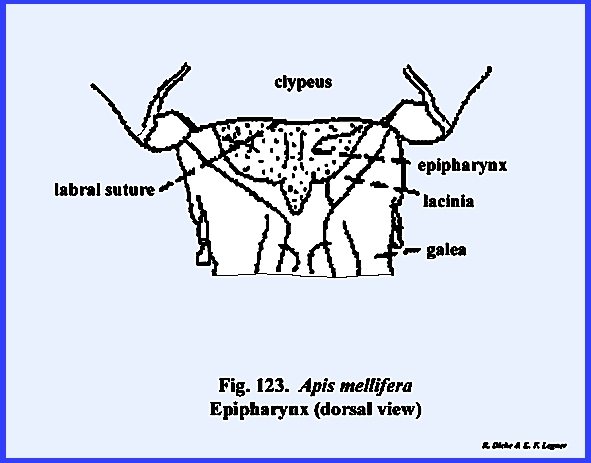
surface, and is probably sensory in function. The inner lobe is the lacinia, which in
contrast with the galea is heavily sclerotized and serves as an adjunct to
the mandibles as a second cutting and tearing instrument. Importantly, its distal end is armed with
three sharp teeth and its mesal margin bears numerous stout setae. Articulation of the opposing maxillae is
on the same horizontal plane as the mandibles. Underlying the mandibles and
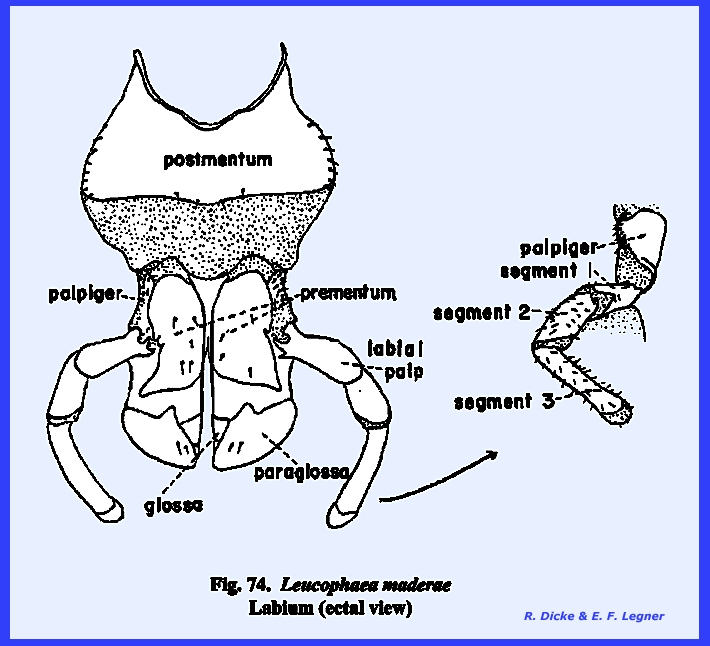
maxillae is the labium. This is a
composite structure which readily can be homologized with the maxillae and
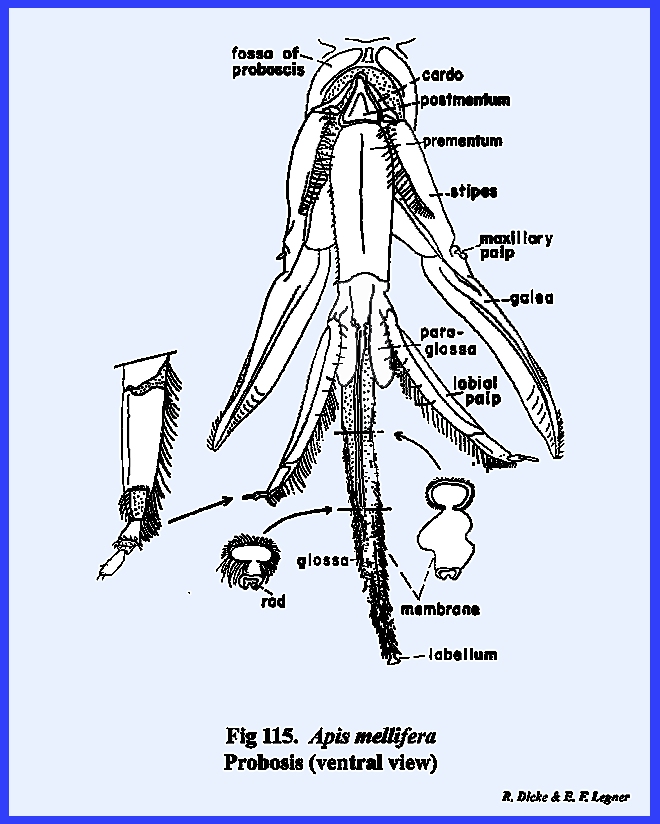
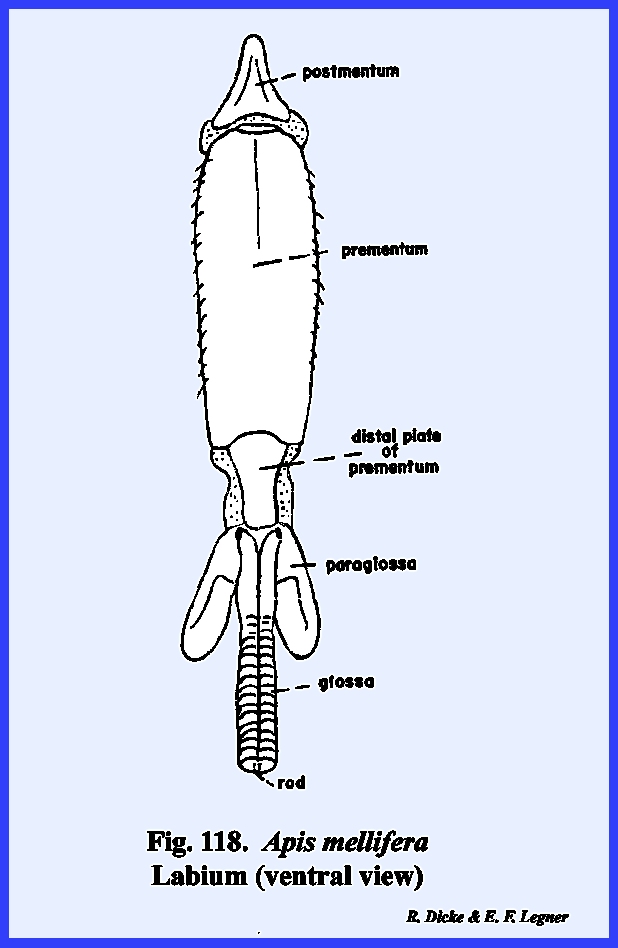
traced ti its origin as a fused pair of typical legs. The broad basal portion of the labium
articulates directly with the cervix, and appears to be closely associated
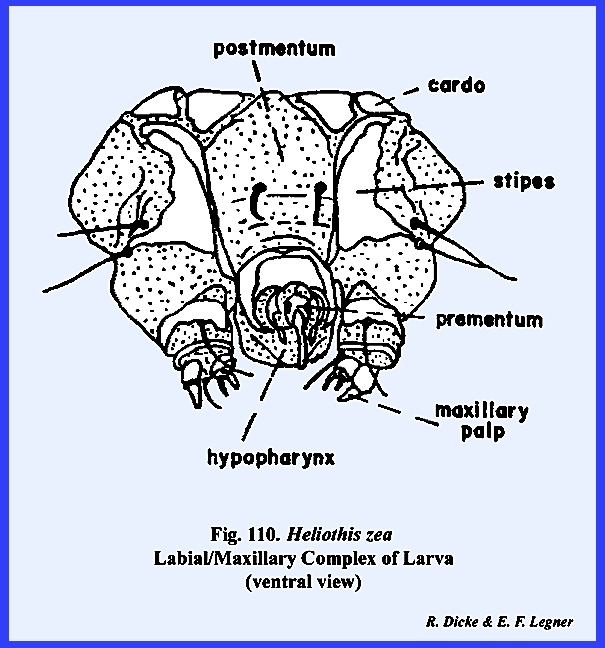
with the postocciput as the sclerite of its origin. The articulatory portion of the labium is referred to as the postlabium and is comparable with the cardo of the
maxilla. Where the postlabium is a
single sclerite as in Leucophaea
maderae, it is usually termed the postmentum (Fig
74). When two
distinct sclerites comprise the postlabium as in Phyllophaga rugosa the most proximal is the submentum
and the distal sclerite is the mentum.
In Leucophaea maderae the
postlabium is composed of a basal sclerite and a distal membranous area. This membranous area probably is not a
true mentum. The distal portion of
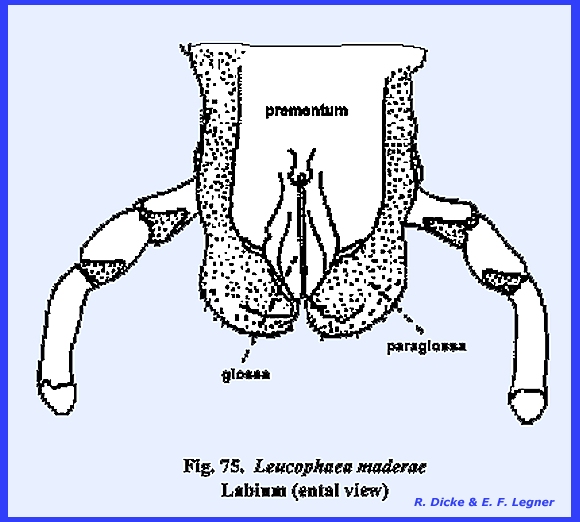
the labium is the prelabium. Its proximal sclerotized area is
the prementum comparable to the maxillary stipes, the inner
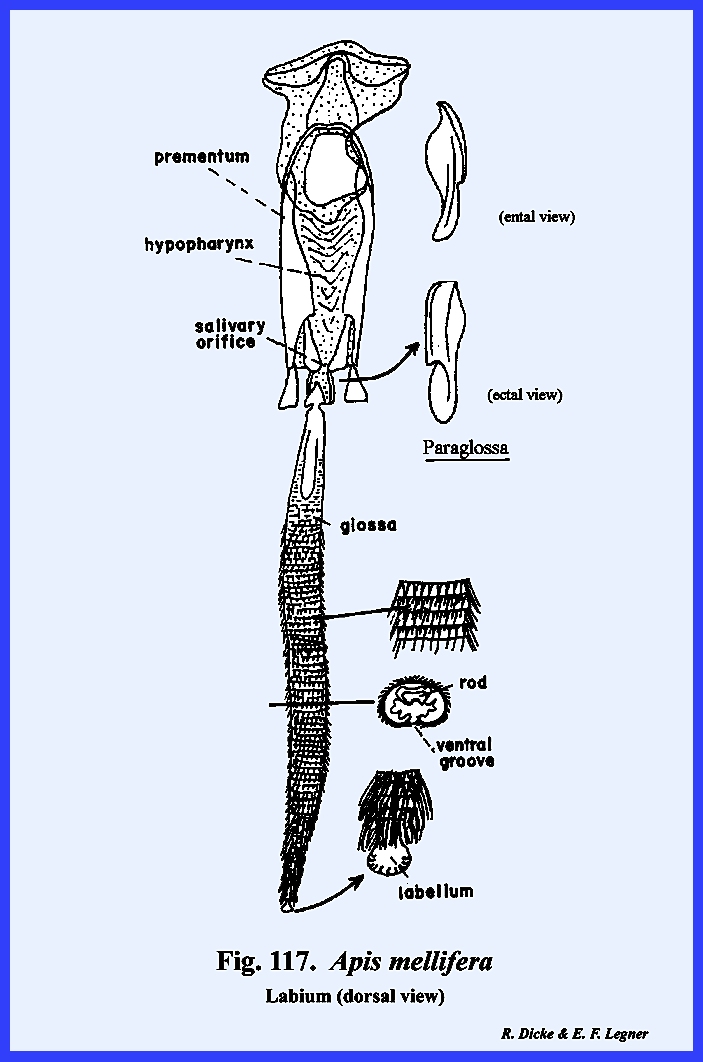
distal lobes are the glossae and the outer lobes the paraglossae (Figs 74 & 75). These lobes are
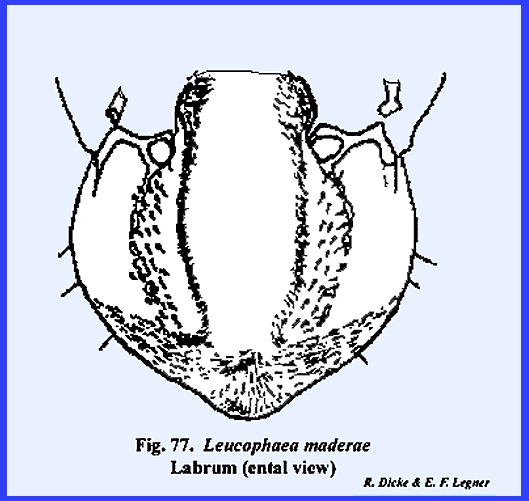
homologous with the galea and lacinia of the maxilla. Development of the glossa and paraglossa
in Leucophaea maderae is best seen
in an ental view of the labium (Fig 75). The deep cleft between the glossa suggests
that the labium originated from a pair of appendages following fusion of the
basal segments. A pair of 3-segmented
palps, the labial palps, is borne by the
lateral margins of the prementum.
These palps articulate with a sclerite (best viewed in Leucophaea maderae from a lateral
view) designated as the palp bearing sclerite or palpiger. The labrum
or upper lip is an integral part of the chewing
mechanism although unlike the labium, it was not modified from
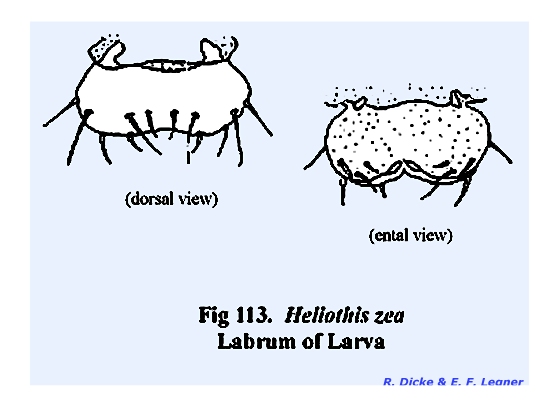
appendages. This ovoid sclerite
probably represents a portion of the old prostomium overhanging the
mouth. The labrum simply serves as an
upper lip for the preoral cavity, connected with the head capsule only along
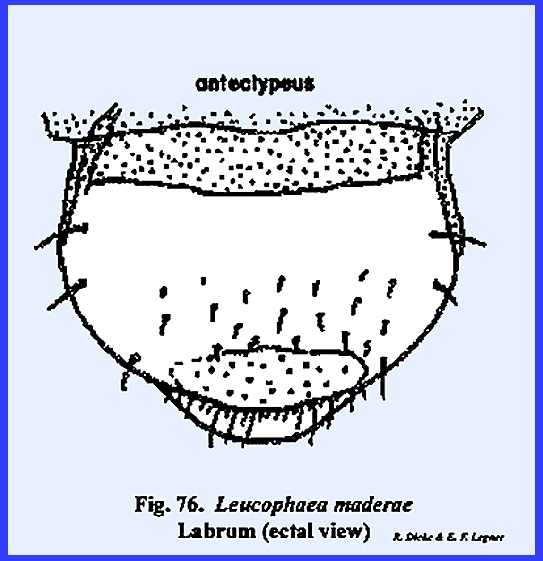
its proximal margin and freely articulating with the clypeus. A mass of sensory pits and setae may occur
on its ental surface as in Leucophaea
maderae (Fig 77) and
particularly in the larva of Phyllophaga
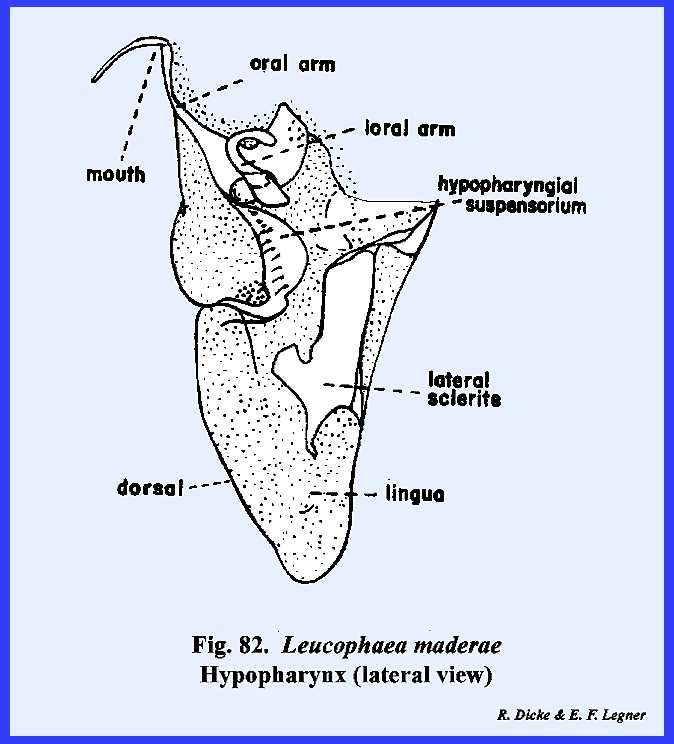
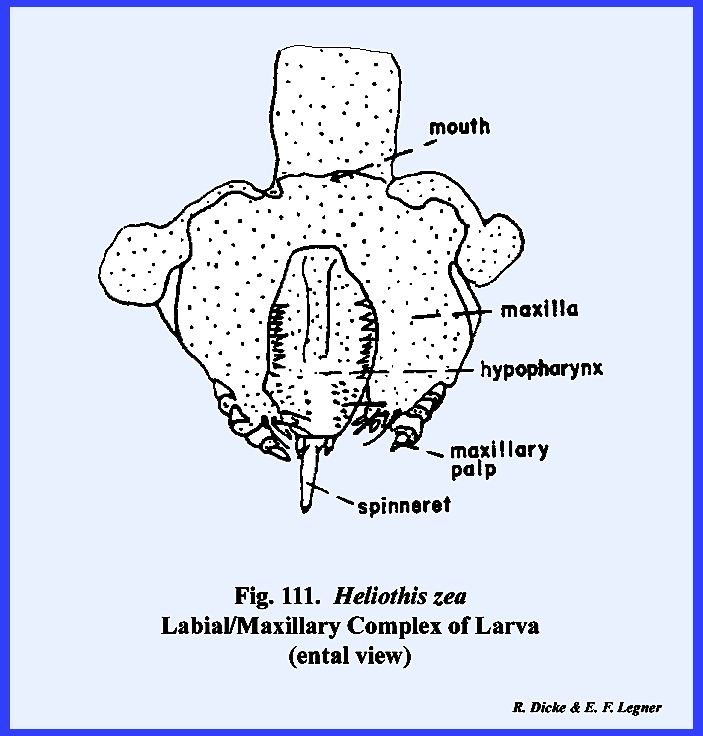
rugosa (Fig 108). The hypopharynx in Leucophaea maderae is a fleshy lobe of
the cranium lying in a median position as a tongue and occupying a large
portion of the preoral cavity (Figs 81 & 82). Its dorsal surface
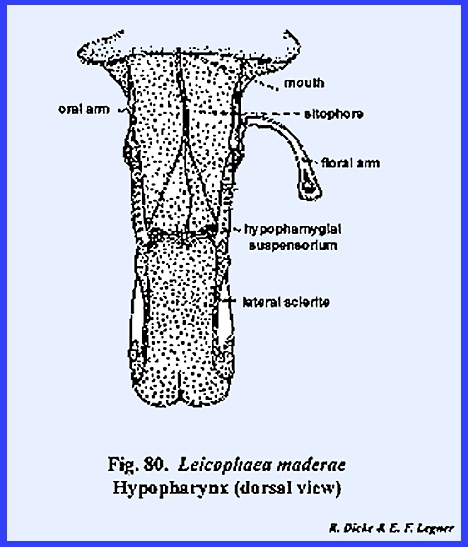
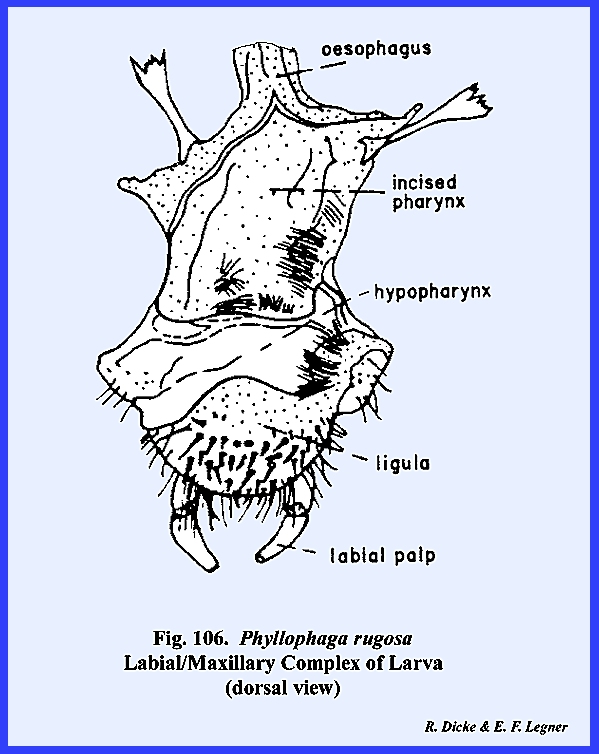
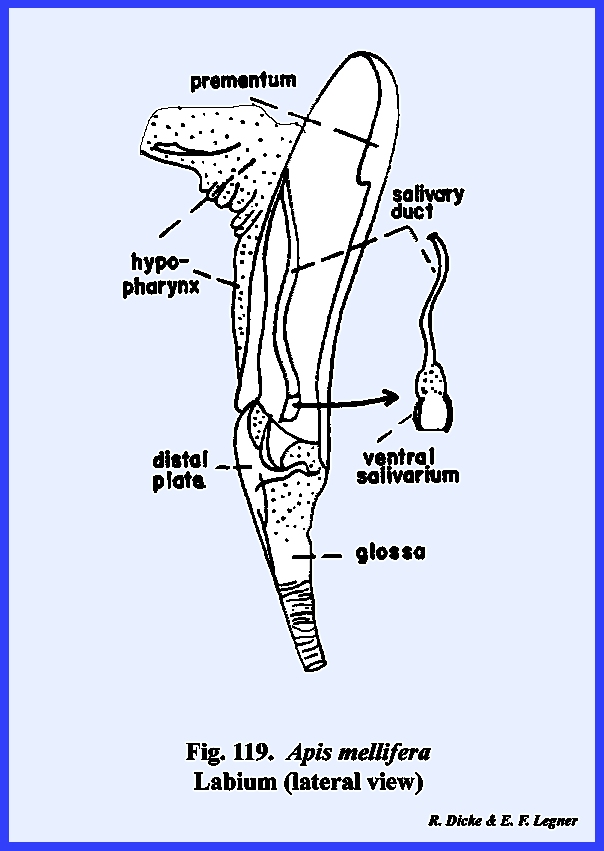
forms the ventral floor of the cibarium, and its grooved base or sitophore leads directly into the mouth (Fig 80). The ventral surface of the hypopharynx
forms the dorsal wall of the salivarium, and the salivary duct empties into
the salivarium at its base. For the
most part, the hypopharynx of Leucophaea
maderae is soft and membranous.
The lateral sclerite and hypopharyngial suspensorium are
sclerites, which articulate with the oral arm, an apodeme
upon which the retractor muscles arising from
the tentorium are inserted. A second
apodeme, the oral arm, provides insertion for retractor muscles arising from
the frons. While the hypopharynx in Leucophaea maderae is a relatively
simple median tongue, this structure may become highly modified in other
forms with mandibulate mouthparts and finally may become an integral part of
the salivary apparatus in insects with haustellate mouthparts. THERMOBIA DOMESTICA: The mouthparts of Thermobia domestica are very similar
to Leucophaea maderae but are
comparatively simple in structure and represent many more of the primitive
features. It was assumed that the
articulation of the primitive mandible was monocondylic. This condition does exist in some of the
more primitive Thysanura, and Thermobia
domestica represents a transitional stage from the primitive monocondylic
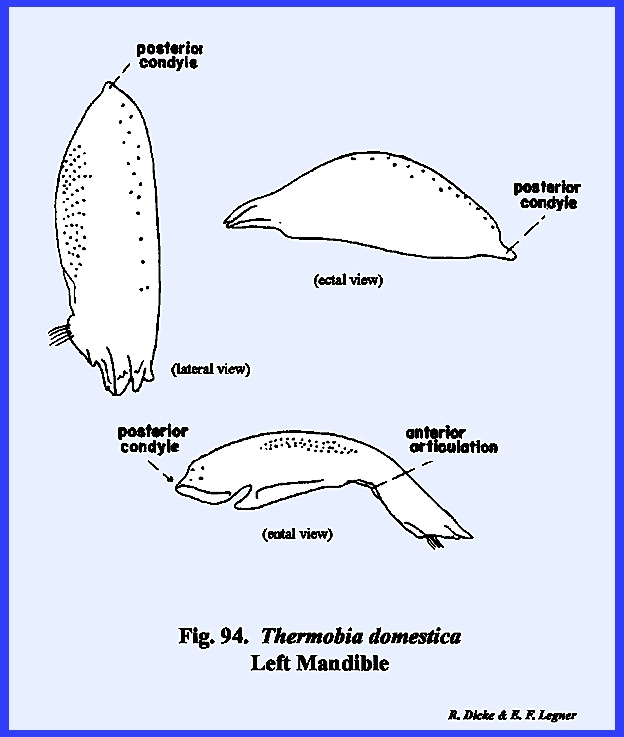
to the more highly evolved dicondylic articulation. The primary point of articulation is the well-developed
posterior condyle. A second but very
weak anterior articulation does occur along the anterior, lateral
margin. While the mandible of Thermobia domestica appears to be a
very weak structure compared with Leucophaea
maderae or Phyllophaga rugosa,
it must have served its purpose well through the millions of years of this
animal's existence (Fig 94). The maxillae
and labium are typical in form although very simple in composition when
compared with Leucophaea maderae (Figs
\, 96 & 98). A
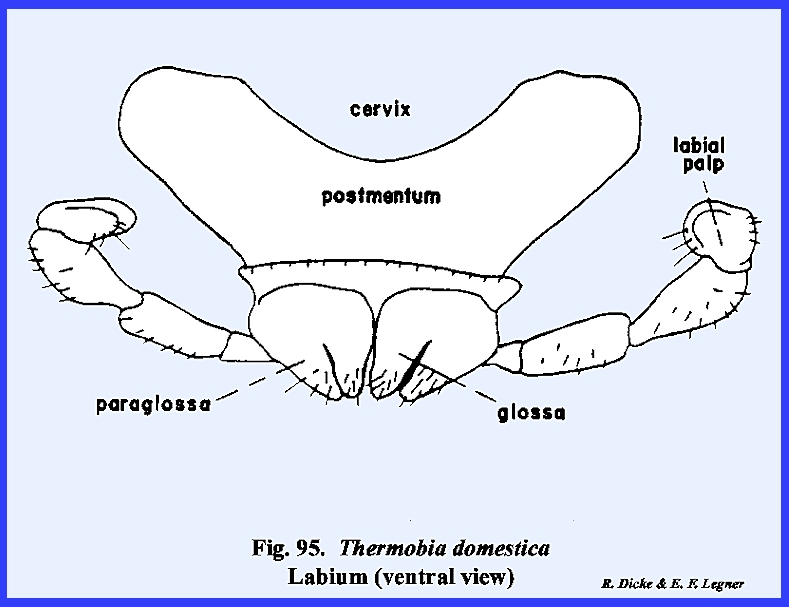
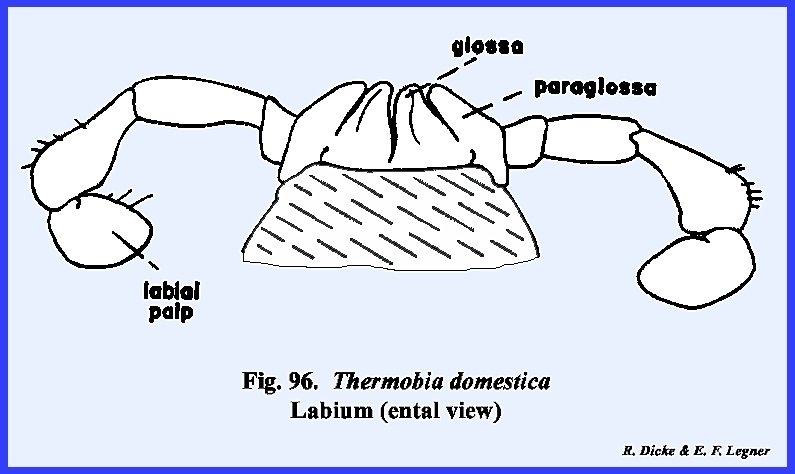
palp-bearing sclerite is absent in both the maxilla and labium. The postlabium is attached to the cervix
by means of a very broad base, and the prelabium bearing the glossae and
paraglossae is greatly reduced.
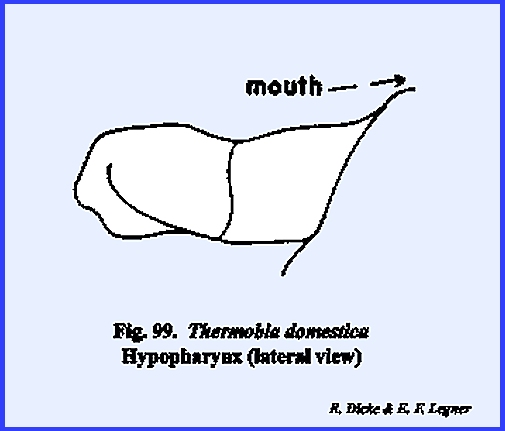
Unlike most insects, the labial palps are 4‑segmented. The hypopharynx is simple and poorly
sclerotized, although there is a distinct division between its basal and
apical aspects (Fig 99). PHYLLOPHAGA RUGOSA: The mandibles of adult Phyllophaga rugosa are blunt, powerful
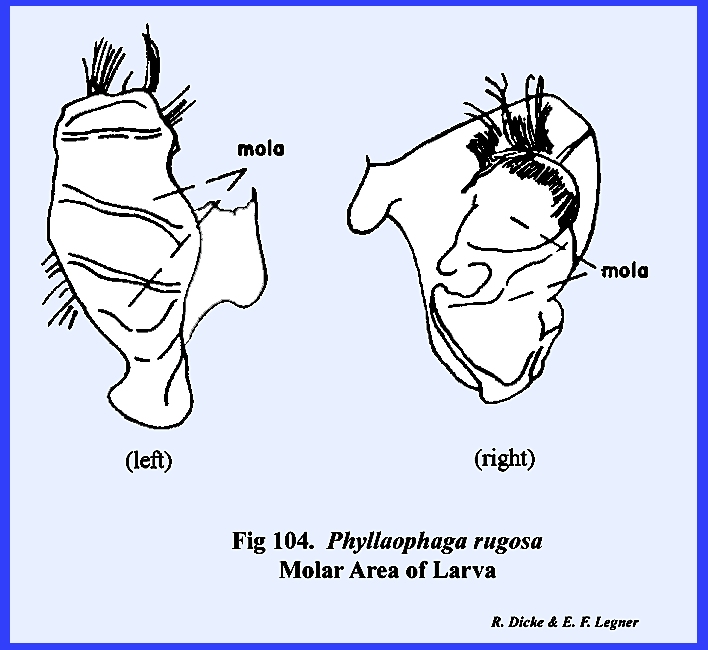
grinding instruments with a broadly developed molar area (Figs 83, 84 & 85). They are dicondylic with a conspicuous
ball‑shaped posterior condyle.
The anterior articulation of the mandible is a pocket, which fits over
a ball‑shaped condyle on the ventro‑lateral margins of the
clypeus. Both of these ball-and‑socket
joints fit so securely that it is difficult to dissect the mandibles from the
head capsule. The adductor apodeme is
very large, and the mandibles are closed by means of powerful muscles. A fleshy ridge or prostheca
extends along the ventro‑mesal margin of the mandible. Its surface is weakly sclerotized, but
does bear a mass of soft, bright yellow setae. The prostheca is a distinct sclerite (absent on all other
insects examined here). It appears to
be homologous with the lacinia of the maxillae. Although the mandibles are heavily sclerotized, they are
covered with setae. The maxillae are typical in form
although the galea is heavy sclerotized and apparently augments the lacinia
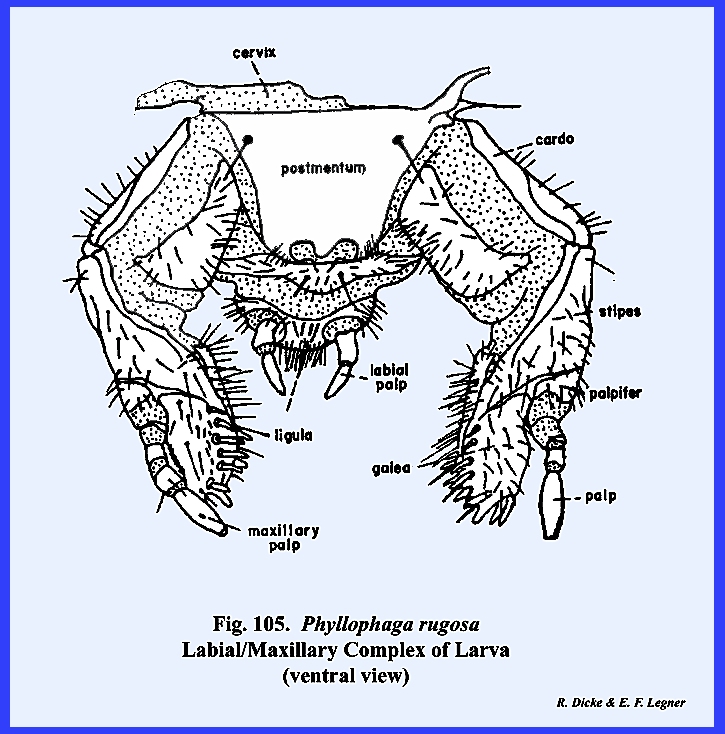
as a cutting instrument (Figs 91, 92 & 93). A large palpifer
bears a 4‑segmented maxillary palp.
The maxillae articulate with the head capsule by means of a groove on
the posterior ventral margin of the gena.
The head of Phyllophaga rugosa
is prognathous (Fig 3), and the
ventral aspect is composed of a gula and an expanded postmentum. The submentum and mentum together are
about 3X as long as the prementum. A
glossa and paraglossa are absent, and the entire premental area is identified
as a ligula. A distinct
palpiger is absent. Unlike Leucophaea maderae and Thermobia domestica, the hypopharynx
is strongly sclerotized on its dorsal surface, asymmetrically lobed and
covered with a mass of setae, and is attached to the labium on its ventral
surface (Fig
87).
The mouth is posterior to the dorsal lobes, and the base of the
hypopharynx is attached to the oesophagus (Fig 90). The labrum is inconspicuous,
underlying the projecting clypeus (Figs 88 & 89). It is deeply cleft on its anterior margin,
and the two lateral lobes are asymmetrical in shape especially when viewed
from the ventral aspect. Also, the
cibarial region is densely clothed behind the labrum with recurved setae. Mandibulate
Mouthparts of Holometabolous Larvae
PHYLLOPHAGA RUGOSA Larva: A generalized mandible occurs in
the grub of Phyllophaga rugosa
which is comparable to Leucophaea
maderae with a similar distal tooth, mola or oral flap. The articulations are strong and well
developed and resemble those of the adult Phyllophaga
rugosa. Also, the molar area is
dentate and asymmetrical, providing a very effective grinding surface (Figs 101, 102 & 103). In all of the species discussed thus far,
the maxillae and labium articulate independently of each other. In the larva of Phyllophaga rugosa and Heliothis
zea these appendages are fused at their base and produce a highly evolved
labial‑maxillary complex. As illustrated in the drawing, the base of
the maxilla is provided with an abductor apodeme, but it should be noted
that the base cannot articulate independently of the labium (Figs 105, 106, 107, 108, 110 & 111). The galea and lacinia are heavily
sclerotized, provided with stout spines and setae, and are completely fused
except for a membranous fissure on the dorsum. In many of the species of Phyllophaga
rugosa, a stridulating field occurs on the ventral surface of the
mandible. This structure is absent on
the mandible of Phyllophaga rugosa
although a row of stout spines or stridulating
teeth line the meso‑dorsal margin of the stipes. It is not known if Phyllophaga rugosa can produce a sound by rubbing these teeth on
the overlying mandibles. A palpifer,
separated from the stipes on its ventral surface, bears a 4‑segmented
maxillary palp. Fused at its lateral
margins with the maxillae is a small labium.
The head of the larval Phyllophaga
rugosa is hypognathous, and the large postmentum articulates directly
with the cervix. The ligula is small
and bears 2-segmented labial palps.
The distal portion of the hypopharynx is an irregular, horny plate
that is fused to the dorsal surface of the labium (Fig. 106) A pair of
apodemes are attached to the walls of what appears to be the pharynx. These may be the oral arms of the
retractor muscles, and the membranous floor of the pharynx may be the basal portion
of the hypopharynx. The labrum is
smooth and ovate on the dorsal surface.
Ventrally, the labrum is developed into a complex sensory field (Fig 108). Sensory pits, grooves and setae are
arranged with sclerotized rods and ridges to form a constant pattern. These structures are arranged in a
constant pattern enabling taxonomists to employ "maps" of the ental
surface for species identification.
An extensive descriptive nomenclature appears in taxonomic literature
that is similar to the previously described chaetotaxy of lepidopterous
larvae. HELIOTHIS ZEA Larva: The mandibles of the herbivorous
larval Heliothis zea are well
developed but quite simple in structure compared with the adult and larva of Phyllophaga rugosa or with Leucophaea maderae. However, these are highly specialized
dicondylic mandibles, and are not
simplified to the extent of the primitive mandibles of Thermobia domestica. The
labial‑maxillary complex reaches its greatest degree of specialization
in Heliothis zea (Figs 110 & 111). Basal
sclerites of the maxillae such as the cardo and stipes are difficult to
identify. The galea and lacinia are
indistinguishable and are represented only by minute papillae. An inconspicuous 2‑segmented papilla
may be the remnant of a maxillary palp.
The labium comprises a soft, membranous postmentum. The prementum is a complex fusion with the
hypopharynx and is probably represented by a sclerotized ring enclosing the
specialized salivary duct or spinneret. Two small papillae may be remnants of the labial palps. The larvae of Heliothis zea are capable of spinning silk, and the salivary
gland has evolved from a gustatorial to a silk‑producing gland (Fig 112). More will be
mentioned about this gland in Section IV.
The ventral view of the labial‑maxillary-hypopharynx complex (Fig 112) illustrates the
extreme degree to which these structures have fused. The labrum is a simple, oblong flap with
little or no sensory modification (Fig 113). APIS MELLIFERA Larva. Larval larvae of Apis
mellifera, unlike Phyllophaga
rugosa or Heliothis zea are
nursed throughout their immature stages of development by adult bees of the
colony. Only a very simple ingestive
mechanism is required for their diet of honey and pollen. The mandibulate mouthparts are
recognizable as such, but are greatly reduced. The labrum is a simple flap, and the mandibles are soft and
weak. Fusion of the maxillae, labium
and hypopharynx has progressed to such a degree that the structures are
undifferentiated and difficult to recognize.
Papillae at the distal aspects of the maxillary lobe and the labium do
not appear to be homologous with palpi.
The grub of Apis mellifera
is also capable of spinning silk produced by the modified salivary
glands. A description of this
spinneret along with that of Heliothis
zea will be reserved for a later section. Haustellate
Mouthparts
The absences of fossil records and
scarcity of example species in existence preclude giving satisfactory clues
to the intermediate steps that may have occurred in the evolution of the
haustellate mouthparts. Since the
feeding mechanisms of Apis mellifera,
Heliothis zea, Oncopeltus fasciatus and Musca
domestica are not comparable; it would appear that their evolution was
completely independent of each other.
An entirely different approach is taken in the elaboration of the
basic mandibulate structures into a sucking tube. All of the mandibulate structures have been preserved in Apis mellifera and Oncopeltus fasciatus, but their
physical appearance is so unlike that of Leucophaea
maderae that they are identified with considerable difficulty. The mandibles in Heliothis zea and Musca
domestica have completely disappeared.
One development appears to be constant for all of these species, and
this concerns the modification of the cibarium into a pumping apparatus
referred to as the cibarial pump. Even in the larvae of Musca domestica, a readily recognizable cibarial pump operates
the complex rasping‑sucking mouthparts. APIS MELLIFERA:
The labrum is a simple flap (Fig 124),
and the mandibles are preserved as in the typical mandibulate form (Fig 125). Although the
mandibles are strong dicondylic structures, they are no longer employed for
tearing and grinding in the ingestion of food. Their function in the drone and queen is obscure, but in the
worker the mandibles are employed for rasping, cleaning brood cells, shaping
wax, and other duties of the hive. A
sexual dimorphism is evident in the form of the mandibles. The ental surface of the worker bee
mandible is a flat and apparently effective spatulate tool. The drone, of course, has little interest
in the labors of the hive, and the hairy mandibles have relatively less
functional form. It has been observed
that the maxillae, labium and hypopharynx in some holometabolous larvae may
combine to form a complex, especially where the salivary glands are modified
for the production of silk. These
same structures in Apis mellifera
have been greatly modified into a sucking tube. The head of Apis
mellifera is hypognathous and the postmentum is a very small triangular
sclerite in comparison with the greatly expanded prementum. The prementum is a completely sclerotized
half‑cylinder (\). This cylindrical structure is completed by
the membranous hypopharynx on its dorsal surface. A sagittal section of the prementum‑hypopharynx complex
reveals the salivary gland and a small salivarium which empties at the apex
of the hypopharynx between two suspensory rods or ligular
arms. At the apex of the
prementum is a small triangular plate described as the distal
plate of the prementum but which may be the ligula (Figs 118 & 119). Two pair of appendages and a median tube
completes the labium, which may be referred to in the literature as the
tongue or proboscis. The large,
segmented outer pair are the labial palps.
The basal two segments are elongated, flat and L-shaped in cross‑section. The distal two segments of the labial
palps are small and typically palpiform. A median pair of spoon‑shaped appendages is the
paraglossae. The salivary duct opens
at the base of the paraglossae, and when the paraglossae are drawn together,
they close ventrally over the base of the median glossa. Although the median tube is referred to as
the glossa, it is an unpaired structure and is only remotely homologous with
the glossae of Leucophaea maderae
or Thermobia domestica. The lumen of the glossa that is ovoid in
cross‑section, is open by means of a longitudinal slit along the entire
ventral surface. A long rod, which is
U‑shaped or deeply grooved on its ventral surface traverses the entire
length of the tubular glossa along its dorsal aspect opening proximally at
the distal plate of the prementum (Figs 115, 116 & 117). The rod is attached to a membrane, which
is usually folded within the lumen of the glossa. Under certain circumstances that are not apparent, the rod may
be extruded from the lumen through the longitudinal slit. The membrane attached to the rod and the
margins of the slit are then expanded into a large sac with the grooved rod
attached along its ventral surface.
At the tip of the glossa is a spoon‑shaped segment given the
descriptive but anatomically incorrect name of labellum (Fig 117). It is
concave on its dorsal surface, and is margined by stout setae recurved toward
the depression. The entire surface of
the glossa is circularly grooved.
Stout, bristle-like setae ring each of the grooves, and the setae are
all pointed anteriorly and alternate in position with each preceding row. The proximal end of the glossa is notched
and appears to swing freely between the bases of the paraglossae. The hypopharynx is actually a part
of the labial complex, and its anterior portion identified by the lateral,
sclerotized hypopharyngial suspensoria is
the dorsal wall of the cylinder partially enclosed by the prementum (Fig 122). The posterior portion of the hypopharynx
is the floor of the bulbular cibarial pump.
It is interesting to note that the median portion of the hypopharynx
before the cibarial pump is expanded or looped outward into a flap or hypopharyngial lobe. A patch of sense organs and paired ducts
or food glands occurs just posterior to the hypopharyngial
lobe. The hypopharyngial lobe should
not be confused with a similar membranous flap given the descriptive name of epipharynx. This
fleshy flap is a special feature of certain Hymenoptera and apparently is
derived from the ental wall of the clypeus.
The maxillae are readily
identified as the flat appendages on either side of the labium (Fig 120). The cardo articulates directly with the
postmentum by means of a V‑shaped sclerite or yoke described as the lorum,
and with the postgena by means of an articulatory flange. The stipes bear a greatly reduced 2‑segmented
maxillary palp, a weakly sclerotized and lobular lacinia, and an elongated
and flattened galea. In cross‑section,
the galea is U‑shaped and similar to the labial palp. In addition, there is a conspicuous median
ridge on its ental surface. The
distal end of the galea is pointed and armed with stout setae. All of the functional details of
this sucking mechanism except for the cibarial pump are by no means
clear. When the flat maxillae and
labial palps are closely appressed around the prementum and glossae, a
tightly sealed food canal is formed as illustrated by
the cross‑section of the proboscis (Fig
116).
The fleshy lobes of the lacinia and the dorsal flap of the epipharynx
serve as sealing devices before the cibarial pump. However, the function of the glossa is difficult to
explain. It is sometimes believed
that the ventral groove of the glossa serves as a salivary channel, although
the salivary duct is dorsad of this slit in the walls of the tubular
structure. The role of the rod is
vague in the description of authors especially when it is distended from the
lumen of the glossa. Possibly the
ventrally grooved rod serves as a salivary channel when it is distended from
the glossa. Saliva could flow from
the dorsal duct ventrally around the bases of the paraglossae even though
this appears to be an illogical development.
The lumen of the glossa with the rod distended might serve as the
actual food canal as far as the bases of the paraglossae. However, the lumen of the glossa appears
to open on the ventral aspect of the labium.
This again appears to be an illogical development for such a highly
specialized apparatus. The food cana1
formed by the galea and labial palps ends a considerable distance from the
labellum, and the glossa, and therefore, must serve as the ingestive device
beyond this point. It has been
suggested that the labellum serves as a lapping device. How liquid food is transferred from the
labellum to the food canal is difficult to visualize. The role of the anteriorly directed setae,
which cover the ectal surface of the entire glossa and margin of the ventral
slit, further confuses a logical explanation of the sucking mechanism. Liquid food does get to the cibarial pump,
either by devious channels or more likely, by an efficient but a poorly
understood route. ONCOPELTUS FASCIATUS: Although the sucking apparatus of Oncopeltus fasciatus is a highly
evolved mechanism, its functional morphology is much clearer than that of Apis mellifera. The long and conspicuous, 4‑segmented
labium is not involved in the elaboration of the actual plercing‑sucking
tube (Fig 132). It is an
ovate cylinder with heavily sclerotized walls and a shallow dorsal groove or
a dorsal gutter. This is such a highly specialized
structure that the elements of a typical mandibulate labium are obscure. The purpose of the labium is simply to
serve as a sheath for the sucking mechanism that lies encased in its dorsal
gutter while at rest. During feeding,
the labium is actually withdrawn and does not enter into the tissues of a
plant host. There is a cluster of
papillae at the distal end of the labium.
These papillae are probably sensory in function and the labium or
proboscis is used as a probe while the insect searches for a desirable food
source. The piercing‑sucking
mechanism is a closely appressed bundle of four, hairlike shafts or stylets. An examination of the musculature at the
base of these stylets suggests that they were modified from typical
appendicular mandibles and maxillae.
In gross appearance, the stylets appear to be a single, hairlike
bristle. However, cross‑section
reveals that four distinct heavily sclerotized and elaborately sculptured
structures are present (Fig 131). Longitudinal
cavities in the stylets demonstrate that they were formed from heavily
sclerotized tubes. The outer pair is
the mandibular stylets which are grooved to fit an inner pair of maxillary
stylets. The mandibles are the
principal piercing stylets. The tips
are pointed and provided with sharp cutting plates and recurved spines for
anchoring the stylets in host tissue
(Fig
134).
The tips of the maxillae also are pointed, but their structure would
indicate that they are secondary to the mandibles as penetrating organs (Fig 135). The cross‑sectional
view illustrates the longitudinal grooves and mortising (= channeling) that holds
the maxillary stylets together, forming longitudinal tubes (Fig 131). The dorsal tube or food canal leads to the cibarium, while
the ventral tube or salivary canal opens into the
salivarium. Mortised joints also hold
the mandibular stylets securely to the maxillae. Although the stylet bundle is securely united, each of the
grooved mandibular stylets may move freely and independently upon the
maxillae on a longitudinal plane.
This forward and longitudinal movement of the mandibles is
accomplished by protractor muscles arising from the mandibular apodeme and attached
to the base of the stylet. The
maxillary stylets as a unit are also protracted by muscles attached to a maxillary apodeme anchored on the posterior tentorium. Penetration of host tissue is
accomplished by the alternate and individual protraction of each mandibular
stylet. When the pair of mandibular
stylets have reached a maximum protraction, the pair of maxillary stylets are
protracted to a position that is even with them, and the cycle is
repeated. Recurved barbs on the tip
of the mandibular stylets serve to hold the entire bundle in position in the
host tissue during each cycle of protraction. When the stylets have reached a desirable feeding site, saliva
is pumped down the salivary canal by means of the elaborate salivary syringe, and liquid food is pumped up the
food canal by means of the cibarial pump.
The highly evolved salivary syringe will be discussed in greater
detail in Section IV. The cibarial
pump (Fig 41) is trough‑shaped and is formed by a
sclerotization of the posterior hypopharynx.
The membranous roof of the cibarium, derived from the ental wall of
the anteclypeus, is provided with numerous vertical spine‑like apodemes
for attachment of the cibarial dilator muscles. The pumping action is simply a raising of the membranous top of
the cibarial trough by the dilator muscles, and a snapping back of the
elastic membrane into a resting position within the trough upon relaxation of
the dilator muscles. The opening or
closing of the trough is accomplished by a forward to backward series of
contractions and relaxations of the dilator muscles because valves are not
provided in the cibarial pump to prevent an opposing backward or forward flow
of fluids. The labrum is a sharply
pointed flexible flap articulating at its base with the anteclypeus and lying
over the dorsal gutter of the entire first basal segment of the labium (Fig 133). This
modified labrum is more than a simple covering flap. Examination of the ental surface reveals a
deep groove which ensheathes the basal portion of the stylet bundle. This is the labral
stylet groove that serves to hold the stylets in position before their
separation in the head cavity. HELIOTHIS ZEA: The proboscis of Heliothis zea
illustrates considerable simplification in the formation of a sucking
tube. The long, coiled tubular
structure bears a superficial resemblance to the ensheathing proboscis of Oncopeltus fasciatus, but close
examination reveals that the proboscis of Heliothis
zea is actually the sucking tube and that it is not a modification of the
labium. In cross‑section, the
proboscis is composed of two ovoid cylinders, deeply grooved on their mesal
surface, and fitted together by mortise joints so that the longitudinal
grooves form a channel or food canal (Figs 126 & 130). Examinations of the basal structures on
each half of this proboscis provide satisfactory clews to its origin. Two articulatory sclerites can be readily
identified as the cardo and stipes.
Identification of the proboscis as a modification of the maxillae is
assured by the inconspicuous, 2‑segmented maxillary palp borne by the
stipes on its ventral margin. The
long, sucking tube must, then, be one of the maxillary endites, and each half
of the tube is usually identified as the galea. At the base of the proboscis, the galea are divided and the
food canal empties through a narrow canal into a large, bulbular cibarial
pump (Fig
127).
The cibarial pump is provided with two sets of dilator muscles as
illustrated by the sagittal section of the head. A small set attached to the head capsule dorsad of the labrum
are probably the true cibarial dilator muscles arising from the clypeus. A large set of muscles identified as the frontal muscle is probably the pharyngeal dilator
arising from the frons. Apparently,
the pump is a composite structure involving both the cibarium and the
pharynx. If this interpretation of
its morphology is correct, then the facial area identified as the frons is
actually a composite sclerite involving both the clypeus and the frons. The salivary gland empties into the narrow
channel connecting the food canal and the cibarium. The surface of the galea is lined with minute, irregularly
parallel ridges. These sclerotized
r1dges or annulations give the proboscis its
spring like characteristic that retracts the structure while at rest into a
tightly rolled coil. The mechanism involved in the
expansion of this coil during feeding is not entirely clear. This feat is probably accomplished by
muscles within the lumen of the galea, which change its shape upon
contraction. When the dorso‑ventral
muscles contract, the ventral surface of the galea is flattened
considerably. This reduces the volume
of the lumen, and fluids within the cavity of the galea are compressed. A basal constriction of the lumen prevents
a back flow of fluids. A compression
of fluids within the lumen may unroll the coil and extend the proboscis in
much the same manner as uncoiling a cylindrical paper toy by blowing air into
its cavity. In the dorsal view of the
proboscis, cross‑sections of the proboscis illustrate this change in
the shape of the galea. The basal
portion of the proboscis of the specimen used for the drawing was
curved. The cross-section of the
ventral surface is deeply grooved.
The distal portion of the proboscis was distended or
straightened. In the cross‑sections
of this portion, the ventral surface is flattened and the volume of lumen is
appreciably reduced. The two sections
of the proboscis are separated at the distal end. The ectal surface is covered with stout setae and minute,
sclerotized protuberances. The tip of the proboscis then, is
provided with an abrasive surface for rasping plant tissue prior to feeding
upon the exudate. All of the other
typical mandibulate parts are reduced or wanting except for the conspicuous
labial palps. These palpi are 3‑segmented
and covered with a mass of setae. The
labium itself is greatly reduced to a simple oblong postmental sclerite
articulating with the cervix, and a plate‑like prementum occupying the
ventral aspect of the head and bearing the labial palps (Fig 128). The mandibles are wanting, or possibly
they are represented as vestiges by two sclerites arising at the lateral
margins of the labrum (Fig 129). These flap‑like sclerites, covered
with stout setae, are pilifers of descriptive entomologists. The labrum is a small flap at the base of
the proboscis. A fleshy distal tip of
the labrum may serve to cover the food canal as it extends into the
cibarium. There appear to be no
remnants of the hypopharynx. MUSCA DOMESTICA:
The fleshy proboscis of adult Musca domestica is a composite unit
that is entirely different from any of the other haustellate structures that
have been mentioned. Only the
sclerites associated with the cibarial pump can be identified with certainty,
and it is necessary to use many descriptive terms to identify structures and
areas of doubtful anatomical origin.
The entire proboscis resembles a stubby, foot-like organ when it is
protracted for feeding (Fig 137). In this position,
there are three distinct regions: 1) a comparatively soft, cone‑shaped
basal region or rostrum, 2) a cylindrical median region
or haustellum, and 3) a distal pair of fleshy lobes forming
the foot or labella.
When at rest, the haustellum is partially retracted within the rostrum
and is folded anteriorly upon it, while the ventral surface of the labella is
tipped upward and posteriorly from its horizontal feeding position (Fig 50). The base of the proboscis or the
rostrum is largely membranous except for a large U‑shaped anterior
sclerite identified as the clypeus, and two small lateral sclerites or
maxillary plates bearing unsegmented palpi which are the maxillary palps (\). The maxillary plates may be simply
remnants of the maxillae or a palpifer.
It appears, then, that the rostrum is a composite structure involving
elements of the cranium and the maxillae.
While the maxillary palps cannot be identified with certainty, there
is little doubt about the identity of the clypeus although its appearance is
quite unlike that of the typical mandibulate Leucophaea maderae.
Internal dissection reveals that the dilator muscles of the cibarium
are attached to lateral invaginations of this U‑shaped sclerite. These invaginations or apodemes are
referred to as the lateral plates best seen in a
sagittal section of the head (Fig 138). Since it was concluded that the cibarial
muscles always arise from the clypeus, the U‑shaped
sclerite and its apodemes must be the true clypeus considerably removed from
the head capsule. The cibarium is a
sclerotized trough, and that a membrane lying in this trough is the actual
pumping diaphragm that is operated by large muscles laterally attached to the
lateral plates or apodemes of the clypeus (Fig 144). The
haustellum is a fleshy cylinder, which is entirely membranous except for a
posterior plate descriptively identified as the thecal
sclerite, and a sclerotized dorsal groove or labial gutter
(Figs 140 & 141). Overlying
the labial gutter is the labrum. The
labrum is ovoid in cross‑section and deeply grooved on its ental
surface (Fig
139).
The hypopharynx is stylet‑like in form and underlies the labrum
and lies within the gutter. The
dorsal surface of the hypopharynx is depressed and the lumen is a
longitudinal tube (Fig 138). The salivary
gland provided with a pumping mechanism or syringe
empties directly into the tubular hypopharynx. The hypopharynx of Musca
domestica, then, not only forms a salivarium but completely encloses it
as well. With the hypopharynx closely
appressed to its ental surface, the longitudinal groove of the labrum forms a
short sucking tube, leading from the labellum to the cibarial pump. Two rod‑like apodemes, the labral apodemes, attached to strong muscles
articulate the labrum at its base.
Lying between the labral apodemes is a narrow canal leading to the
cibarium. A small plate identified as
the hyoid sclerite apparently distends
this narrow passage, which serves as the mouth. The sucking tube formed by the labrum and hypopharynx does not
actually penetrate a food medium. It
simply serves to conduct fluids from the labellum, which is the actual
collecting mechanism, to the cibarium.
Also, saliva is conducted to the distal end of the labial gutter by
the salivary canal enclosed within the hypopharynx. The labella are fleshy lobes
forming a foot‑like pad at the distal end of the proboscis (Fig 142). It is assumed by morphologists that these
lobes are modifications of labial palps although their resemblance to palpi
appears quite remote. When the
proboscis is at rest, the ventral surfaces of the two-pad‑like labella
are folded mesally as illustrated by the frontal view of the proboscis. During feeding, the labella are broadly
expanded and directly contact the food source. The labellum is deeply incised on its anterior margin, and the
incision corresponds with the groove of the labial gutter. A V‑shaped sclerite, the discal sclerite, margins the apex of this labellar
incision (Fig
143).
The orifice enclosed by the discal sclerite is the prestomum or
functional mouth. Food passes through
this orifice and directly into the food canal formed by the labrum and
hypopharynx. A series of tubes
transversely lines the labellar lobes and empty into large collecting channels which parallel the
discal sclerite. These tubes are
referred to as the canaliculi, or pseudo tracheae
since they remotely resemble the tracheal tubes of the respiratory system (Fig 143). Sclerotized
rings distend the canaliculi. The
rings are incomplete on the ectal surface, and they alternately terminate in
a U‑shaped fork. A longitudinal
slit occurs along the ectal surface extending between the expanded ends of
the sclerotized rings. This slit is
best demonstrated by a cross‑sectional view of the canaliculus
(Fig 143). The dorsal
slit is believed to be further expanded between the U‑shaped expansions
of the rings. Sclerotized rings also
distend the collecting channels, but these tubes apparently do not have a
dorsal slit. While the mechanics of the sucking
apparatus described for the rostrum and haustellum is not difficult to
understand, the function of the labella is quite vague. It is apparent that the canaliculi serve
as collecting tubes since the dorsal slit permits fluids to enter the hollow
tube by capillarity. The fluids would
then "flow" into the collecting channels. It has been assumed that the collecting channels (and the four
canaliculi independent of the collecting channels) empty directly into the
prestomium. However, these tubes,
which are attached to the discal sclerite, narrow sharply before their
attachment. Dr. Robert Dicke, who
performed most of the initial dissections for the illustrations, states that
he has not been able to demonstrate an orifice through which liquids could
flow into the prestomium. But, we
must assume that liquids (and particles of solid food) do flow from the
canaliculi into the prestomium from where the sucking apparatus of the
haustellum and rostrum deliver the food material to the oesophagus. Musca
domestica apparently can scarify a food medium to some extent since five
sclerotized teeth are anchored on the discal plate on the mesal margin of
each labellum. These minute plates
are the prestomal teeth (Fig 143
When the labella are pressed against a surface and presumably rotated, the
prestomal teeth may serve as a cutting and rasping device. A unique sucking and rasping device has evolved in the maggot
or immature stage of Musca domestica
that is only remotely comparable with that of the adult. Previously, it was established that the
typical head region is incompletely developed in the larva. In fact, all of the cranial structures of
the adult are represented only by primordial cells deeply invaginated within
the body cavity. The functional head
of the maggot is provided with rasping structures that are unlike those of
any other insect form. Within the
thorax is a large, trough‑shaped apparatus (best seen in a dorsal view)
which in lateral view appears to be a pair of flat sclerites with prominent
posterior expansions (Fig 145). Descriptive
taxonomists most commonly refer to this structure as the cephalopharyngial skeleton. The nature of this structure is not
apparent in taxonomic preparations cleared in a caustic solution. The first clew to its identification in
an uncleared dissection is the attachment of the oesophagus to its posterio‑ventral
aspect. A cross‑sectional
examination will immediately identify it as a cibarial pump (Fig 147). Actually, the cibarium of the larva is
comparable to that of the adult. The
lateral walls of the trough to which the strong, dilator muscles are attached
are similar to the lateral plates of the adult. The floor of the larval cibarium is homologous with the
posterior wall of the adult.
Certainly, the pumping diaphragm, its position in the trough and the
attachment of the dilator muscles is the same in structure as they are in the
adult cibarium. If these homologies
are correct, the anterior aspect of the cibarium must be the clypeus, and the
lateral walls of the maggot cibarium must be the apodemes or invaginations of
the clypeus, which were identified as the lateral plates in the adult. In the illustration (Fig 145), an anterior prominence on the cibarium of instar-III
is identified with some reservation as a labrum. In instar-I this is a distinct sclerite separated from the
cibarium; it is distinct but fused in instar-II, and finally becomes an
integral part of the cibarium in instar-III.
Although the cibarium is greatly reduced in the first instar, it is
comparable in form for all of the three larval stages. The feeding structures anterior to the
cibarium appear to have no counterpart in other more primitive forms. A hook‑like structure, the
mouth hook, protrudes from the functional mouth of the maggot. In Musca
domestica, this appears to be a single structure unlike other muscoid
species. The dorsal view reveals that
the mouth hook is actually a fusion of a pair of hooks, which can be
separated. The two hooks are asymmetrical. The right hook is the larger of the two
and its distal aspect accounts for the bulk of the anterior hook. The left hook is relatively weak and
closely appressed to its companion. A
small sclerite (the dental sclerite of taxonomists) is attached to its
ventral aspect. Intervening between
and articulating with the mouth hook and the cibarium is a small sclerite
identified as the hypostomal sclerite (Fig 146). This sclerite is notched at its posterior
margin to accommodate and support the salivary gland. The hypostomal sclerite is grooved on its
dorsal surface to provide a salivary canal.
The mouth hook and hypostomal sclerite are progressively modified in
design from the first to the last instar.
Additional sclerites of unknown morphology also occur in
instar-II. Since the head capsule in
the maggot is retarded in development, it is probably unwise to attempt to
homologize beyond the cibarial apparatus.
It is very unlikely that the mouth hooks are homologous with mandibles
or the hypostomal sclerite with a hypopharynx. Certainly, these are not precursors of any adult structures,
and they are completely discarded during the pupal instar. The mechanics of the apparatus are
not difficult to visualize. The mouth
hook and hypostomal sclerite are enclosed in a membranous sac, which serves
as a functional mouth or atrium.
The mouth hook may be protracted and withdrawn by which action it
serves to scrape a food medium.
Fluids and particles of solid food are drawn into the atrium by the
sucking action of the cibarial pump, mixed with saliva, and finally pumped
into the oesophagus. SECTION IV - ORIGIN OF THE
PRINCIPAL BODY REGIONS
Dr. Robert Dicke dealt in
considerable detail with evolutionary aspects of the insect body
development. The following is derived
largely from his account to students at the University of Wisconsin. Fossil records indicate that insects were
probably as numerous on earth 150 million years ago as they are today. But the time of their origin, or the
stages through which they progressed in their evolution, are obscure. There is some fragmentary evidence that
Collembola-like arthropods may have occurred in the Devonian geological age
ca. 350 million years ago. Unquestionable
fossil records have been recovered from rocks dating to the Upper
Carboniferous Age of ca. 250 million years ago. Even at these ancient times, insects had their wings fully
developed. Outgrowths of the
prothorax on some of these fossil forms do provide evidence that wings may
have evolved from similar paranotal lobe structures. It certainly appears that all of the
important evolutionary changes in insects were completed before the beginning
of the Permian Age, dating from 215 million years ago. Because of the incomplete palaeontological
records, most evidence on the origin of insects must be drawn from three
sources of study: 1) a comparative morphology of ancient and modern
arthropods, 2) a comparative morphology of insects as we know them today, and
as they relate to fossil forms recovered from the deposits of carboniferous
and subsequent geological ages, and 3) the study of embryonic forms of
present‑day insects. The Trilobita are ancient
arthropods that lived about 550 million years ago, appearing as fully
developed animals in early Cambrian rocks and continuing to exist beyond the
Carboniferous Age and into the Permian.
Living as companions with the trilobites were the now extinct Eurypterida
and the Xiphosura or horseshoe crabs of which living representatives may
still be found along coastal waters.
Comparing structures and systems of these ancient forms with
present-day arthropods such as the Arachnida, Crustacea, Chilopoda, Diplopoda,
Pauropoda and Symphyla has allowed for speculation on the pattern of
development that may have occurred in the evolution of insects. Much of the theory on the origin and
development of locomotory appendages and the organization of the principal
body regions may be derived from such a study. The antiquity of structures such as the compound eye, antennae
or chewing mouthparts helps to establish whether these organ systems are
relatively primitive (have occurred early in phylogenetic history) or specialized
(of relatively recent origin). A study of the comparative morphology of ancient and
modern insects further establishes relatively primitive or specialized
structures. Of the present‑day
insects, cockroaches or the Blattoidea are probably the earliest occurring
forms. These appeared in great
numbers in the Upper Carboniferous of about 250 million years ago, and are
essentially the same morphologically as the forms that have adapted
themselves to such an intimate relationship with our present human
civilization. The morphology of the
roaches may then he contrasted with the Hymenoptera or Diptera, which
probably appeared in the Jurassic Age some 95 million years later. A study of internal morphology furnishes
further clews on the probable evolution of the principal body regions. For example, the organization of the
central nervous system indicates the probable metameric organization and the
fusion of these metameres into the main body regions of a typical insect. Embryologists have suggested that
"ontogeny [development of an individual]
repeats or gives evidence to phylogeny [history of a race]. Therefore, a study of embryonic forms reveals the abortive
development of structures long discarded by the individual during its
evolution into a present‑day adult form. Usually these studies are much more fruitful in the relatively
primitive forms such as the Collembola or Orthoptera. Embryonic phylogeny has become obscure in
groups in which a highly specialized form of metamorphosis has evolved such
as in the Holometabola. These studies
do provide sufficient evidence to support such theories as a head development
involving the fusion of five metameres, and suggest the probable occurrence
of such appendages as a second pair of antennae. STAGE
I. WORM‑LIKE PROTOTYPE
The phylum Arthropoda is probably most closely related to the
phylum Annelida, and phylogenists have generally agreed that the arthropods
and annelids probably evolved from a common prototype. A hypothetical depiction of such a
prototype would be a 2O‑segmented, worm‑like animal in which the
mouth was situated posterio‑ventrally in the first anterior metamere
generally designated as the archeocephalon or
prostomium (Fig148). In this concept, the prostomium and all of
the postoral metameres were relatively uniform in size and composition. The body served primarily to house the long
intestinal tract which terminated as an anus in the 20th metamere designated
as the periproct. However, the
prostomium and periproct probably should not be designated as true metameres. The prostomium may be considered as a
sensory lobe or "head" derived from the first anterior metamere,
while the periproct may be considered simply as a lobe bearing the anus as
an outgrowth of the last metamere. In
any case, the composition of the animal may be depicted as a series of
uniform, undifferentiated divisions coextensive with the intestinal
tract. It may also be assumed that
this animal could have been the prototype for the earthworm as well as for
the cockroach. STAGE
II. DEVELOPMENT OF APPENDAGES
Probably the first major change in
development, which separated the arthropods from the annelids, was the
acquisition of paired appendages by all of the major divisions of the body (Fig 149). Latero-ventral protuberances of the body
wall probably developed uniformly on all of the metameres from 3 to 18, and
were employed for locomotion. Appendages developing on the
prostomium, and on the first post‑oral and the 19th metameres were
sensory in function. The anterior
sensory structures are designated as antennae while the posterior pair
probably were the cerci of present‑day primitive
insects. The antennae and cerci of
present day insects cannot be readily homologized with typical walking
legs. Therefore, it may be assumed
that while all appendages arose as simple outgrowths of the body wall,
antennae and cerci evolved directly as sensory structures and were not
modified from ambulatory appendages at a later stage in development. Well-developed filiform antennae (primary antennae) and cerci certainly occurred
early in the evolution of insects.
Although there are no known insects including extinct species bearing
two pairs of antennae, there is sufficient evidence that the second antennae
or postoral pair may have developed in such an early prototype. In the embryos of certain primitive
species, a reduced second antennal protuberance may be identified, but this
structure is completely suppressed before completion of embryonic
development. In a few adult forms,
small lobes situated before the mandibles may be vestiges of such
appendages. The postantennal
appendages in the Crustacea are frequently referred to as structures
homologous with the hypothetical second antennae of insects, although these
appear to have been modified at a later period from leg‑like
appendages. The evidence proposed to
support the existence of a prehistoric second pair of antennae is weak,
although the supposition cannot be completely ignored. Photoreceptors probably evolved
early, and it should be recalled that well developed compound eyes occurred
in the Trilobita, Eurypterida and Xiphosura during the early Cambrian
Age. It has sometimes been assumed
that these eyes evolved from a pair of appendages in addition to the
antennae, and therefore there is the probable existence of an additional
metamere in the head complex. The
stalked eyes of the crayfish Cambarus are frequently referred
to in support of this theory. Since
the dioptric apparatus of the eye is simply a
modification of the integument, development from an existing appendage would
appear to be an illogical step in their evolution. Ocelli also appeared in these early Cambrian forms, and it
would seem equally illogical that these were evolved from appendages and
would account for still other additional metameres. STAGE
III. CEPHALIZATION AND
DIFFERENTIATION OF APPENDAGES
The term cephalization
in this phase of development implies the coalescence or unification of
sensory structures and the mechanisms designed for food ingestion into a
composite unit usually identified as the head (Fig
150). Unification of sensory structures
would be a logical first step, combining the prostomium bearing the primary
antennae and photoreceptors with the first postoral metamere and its second
antennae. This primitive head,
combining only the principal sensory structures, is referred to as the
protocephalon. As this prototype
became a more highly organized animal, the anterior appendages were utilized
and subsequently modified to aid in the ingestion of food. The locomotory appendages of metameres 3,
4 and 5 gradually evolved into the three principal appendages of the
mandibulate mouthparts. Along with
this specialization of appendages it may be assumed that a coalescence of the
metamere bearing them probably occurred, bringing the feeding structures
closer to the mouth. This combined
region is designated as the gnathocephalon.
Prior to the development of the gnathocephalon, the second antennae
may have served both a sensory function and as an ingestion device. As the appendages of the gnathocephalon
developed into the more efficient mandibles, maxillae and labium as they are
known today, the utility of the second antennae decreased and the structure
was eventually discarded. A study of the central nervous
system leaves little doubt that at one time each metamere was innervated
independently by a central nerve center or ganglion. Eventually these ganglia were united by
the interconnected ventral nerve cord. Examination of this system in present‑day
insects provides evidence of the probable divisional composition of the
prototype. The composition of the
gnathocephalon by the coalescence of three metameres appears to be
reasonably well established since the suboesophagial
ganglion that innervates the mandibles, maxillae and labium appears to
be a composite or three ganglia.
However, there is some question about this three‑segmented
gnathocephalon in relation to the superlinguae
(or paragnatha) which in certain primitive insects
appear as paired lobes associated with the hypopharynx. There is some evidence that these lobes
may be the vestiges of a pair of post mandibular appendages, which may have
united to form between them the median hypopharynx. The suboesophagial ganglion does innervate the hypopharynx. If this evidence is sufficient, it could
be assumed that four metameres were involved in the composition of the
gnathocephalon. The protocephalon is
that portion of the definitive insect head, which is innervated by the supraoesophagial ganglion. This ganglion is composed of three
distinct parts: the proto cerebrum with its large optic
lobes, the deutocerebrum which innervates the
antennae, and the tritocerebrum which innervates the
labrum and is connected with the frontal
ganglion and the stomodaeal
nervous system. It appears
that the origin of the tritocerebrum was postoral
since the commissure uniting the two lobes of this ganglion, the suboesophageal commissure,
loops around the oesophagus and lays ventrad and posterior to the mouth. This ganglion probably represents the
first post oral metamere which probably bore the second antennae. The proto cerebrum and deutocerebrum
definitely are preoral and probably innervated the sensory structures of the
prostomium. As was previously
suggested, it seems unlikely that the compound eyes evolved from appendicular
appendages. The proposal has been
made that the proto cerebrum is simply an expansion of the original
prostomial ganglion to accommodate the highly evolved compound eyes. It was assumed in Stage II that
all of the appendages of metameres 3 through 18 were utilized as walking legs
similar to those of the Chilopoda and Diplopoda. In Stage III, the legs of metameres 3, 4 and 5 were modified
into mouthparts, and still other specializations of these appendages probably
developed in other metameres of the body.
Segmentation of the appendages would result in a much more effective
walking leg. Development of the legs
on metameres 6, 7 and 8 and a corresponding reduction of legs on the
posterior metameres would also increase the efficiency of the ambulatory
mechanism. The primitive genital pore of the female probably was situated on the conjunctival membrane behind
the sternum of the 15th metamere.
However, in modern insects this pore is found on the 16th (8th
abdominal) wherever there is a special mechanism provided for
oviposition. There is good evidence
that the prototype of present‑day insects was equipped with an ovipositor, and that two pairs of walking legs were
modified into the valvulae of this structure. A corresponding modification of the
appendages on the seventeenth metamere (ninth abdominal) of the male evolved
into a clasping device of the copulatory mechanism. Since the metamere of Stage II, visualized as an inflexible
ring retarded motion, a longitudinal suture may have developed dividing each
metamere into a dorsal tergum and a ventral sternum. STAGE
IV. DIFFERENTIATION OF THE PRINCIPAL BODY
REGIONS
A division of the body into
specific regions or tagmata was a logical development following specialization
of the appendages (Fig 151). Fusion of the protocephalon with
its sensory organs and the gnathocephalon with its specialized appendages
surrounding the mouth comprised the head tagma where
the division of function was then related to sensory perception and food
ingestion. The appendages of
metameres 6, 7 and 8 evolved into an efficient walking mechanism along with a
corresponding expansion and elaboration of the metameres. Important developments were of the broad
lateral surfaces, the pleurae, for better manipulation of the leg base. Lateral expansions of the terga probably
occurred early in the evolution of the Pterygota. These were the paranatal lobes that
may have been the precursors of wings on the 7th and 8th metameres. In this area of the body, the thoracic tagma, division of function was related to
locomotion. The remaining metameres
were involved in relatively little elaboration since their appendages
gradually lost their locomotory function and were eventually discarded. Only the appendages of the 16th and 17th
metameres were retained and developed into a functional ovipositor or
copulatory mechanism. With loss of
locomotory or sensory function, the terminal metameres were reduced in size
or tended to coalesce. This area of
the body evolved into the abdominal tagma, and its
division of function was related to reproduction and to the centralization of
such visceral systems as the digestive tract, the principal respiratory
tract, circulation, storage and elimination. It was suggested that present‑day
insects evolved from a 20‑segmented prototype in which function has
dictated the formation of 1) a head of five metameres centralizing sensory
perception and the mechanism for ingestion of food, 2) a thorax of three
metameres designated for a both terrestrial and aerial locomotion, and 3) an
abdomen composed of the remaining 12 metameres which houses the important
visceral systems and serves as the seat of reproduction. SECTION V - COMPOSITION OF
THE CUTICULA
The layer of cuticula which
envelopes the insect body and its appendages serve both as an integument for containing tissues, and as a skeleton by
providing support for organ systems and muscles (Fig
152). It is an exoskeleton since it is
an external structure, but it is also an endoskeleton since the cuticula may
be infolded or invaginated to form internal ridges
and rods. The cuticula may have
served simply as an integument for the worm‑like ancestor of
insects. Modification of the cuticula
into hard plates or sclerites and the expansion of it
into appendages brought about the complex
mechanisms that in insects evolved into the locomotory, sensory, ingestive
and reproductive structures which are unique in the Animal Kingdom. The cuticula comprises three
distinct layers: an outer, thin layer of epicuticle,
a median layer of pigmented and usually hard and inflexible exocuticle, and an inner layer of soft and flexible endocuticle (Fig
152).
A single layer of cells identified as the epidermis secretes these
layers. The cuticula appears to be
composed of irregular horizontal layers.
Minute vertical striations in the cuticula suggest that protoplasmic
strands projecting from the epidermal cells secrete these layers. A thin, fibrous sheet, the basement membrane underlying the epidermis
completes the body wall. The epicuticle is a surface film about one micron in thickness
made up primarily of proteins and lipids.
It is a protective film resisting the effects of such environmental
stresses as excessive humidity and desiccation. One of the basic constituents of
the exocuticle and endocuticle is a polysaccharide polymer commonly known as
chitin and chemically identified as a polyacetylglucosamine. Chitin is a colorless, transparent, soft,
flexible material insoluble in water, alcohol, ether, dilute acids and
alkalies. A second constituent or
groups of constituents are complex, long chain proteins, which apparently
serves as a framework for all other chemicals deposited in the cuticula. The endocuticle is composed primarily of
chitin and unmodified proteins.
Endocuticle, then, is the principal component of areas of the
integument that are soft and membranous and at all points of articulation
between hard plates or sclerites, and metameres or appendicular segments. The exocuticle is composed of the same
framework of chitin and proteins but is usually hardened or sclerotized by a polymerized protein referred to as sclerotin. The
exact chemical nature of the proteinaceous sclerotin and the tanning process
or hardening of this protein is still under vigorous investigation. Exocuticle also is impregnated with lipids
and is variously colored by pigments. The cuticula is seldom smooth, and
microscopic examination will reveal many raised areas, corrugations, ridges
and blunt or sharp projections. Much
of this sculpturing is due to expansions or protrusions of the exocuticula
such as the spines and microtrichia illustrated (Fig 152). These are the fixed, usually inflexible,
non‑cellular processes of the cuticula. Other more macroscopic processes may involve an invagination or
a protrusion (evagination) of the entire body
wall. The apodeme of the illustration
(Fig 152) is such a multicellular process where the
invagination involves not only the cuticula, but the epidermis as well, A
chalaza, which is a descriptive term for an external protrusion, may be
heavily sclerotized (e.g., involves the exocuticle primarily), but its evagination
also involves all of the other elements of the body wall. Some of the epidermal cells may be
greatly enlarged and specialized for the secretion of unicellular processes
instead of a laminated cuticula.
Hollow, tubular setae arise from trichogen cells.
A long, protoplasmic strand arising from the trichogen cell extends
through the formative integumentary cuticula, and lays down around it a layer
of cuticula. When the protoplasmic
strand recedes back into its cell, a hollow tube remains composed primarily
of exocuticle. The cuticula of the
integument provides a circular pocket or alveolus,
and a second specialized epidermal cell, the tormogen
cell, secretes a cuticular setal
membrane at the base of the alveolus.
The seta, then, is firmly seated in the alveolus and is capable of
articulation with the integument by means of the flexible setal
membrane. The tormogen cell
completely surrounds the base of the seta and at least a portion of the
trichogen cell. Additional
specialized cells may be associated with the trichogen and tormogen
cells. A poison cell may
secrete an urticating fluid into the hollow seta. When such a seta is broken in the skin of a predator, the
urticating fluid is released. Some
setae are provided with sensory nerve cells. An axon extending to or
within the setae modifies it into a simple tactile
sense organ. Many of these
tactile setae overcome one of the serious disadvantages of an impervious and
confining integument by providing the insect with a means of contact with its
environment. When a seta is broken
and removed, the circular alveolus remains as a pit. Not all of the pits or punctures that
occur in the cuticula are provided with setae. Many of the pits are simply ducts leading from a gland cell in the epidermis. Secretions from these gland cells simply issue from the pore
and spread over the external surface of the cuticula. Chemoreceptors,
which are simply modified setae, may be found enclosed within a deep
alveolus. A cuticular pit, then, may
simply be the orifice for a chemoreceptor provided with a sensory cell. The depth of the alveolus and the size and
sclerotization of the modified seta enclosed within it will determine the
sensitivity of the chemoreceptor. SECTION VI - ORIGIN OF THE
MOUTHPARTS
The primitive leg of the insect
prototype probably was a simple, tubular expansion of the body wall (Fig
153). Muscles of the body operating at
its base manipulated this structure.
The legs of Cambrian trilobites were fully segmented, and fossils of
the earliest insects demonstrate that segmentation occurred very early in
their evolution. Little evidence on
their evolution is available from embryological studies, however. Therefore, it may be speculated that their
probable evolution was from such a theoretical, tubular evagination. Segmentation of a limb completely encased
in cuticula would increase its efficiency considerably. It may be assumed that this segmentation
was no more illogical than the segmentation or metamerism of the body. The first segment of the leg
probably occurred at its base leaving a basal
coxapodite and a distal telopodite. Further divisions or segments of these two
principal regions are referred to as coxites
and telopodites.
In order to accomplish more effective muscle attachment and
articulation, the coxapodite was subdivided into a
basal subcoxa and an apical coxa. These divisions are still evident in some
of the primitive Apterygota. In the
more highly evolved forms elements of the subcoxa were elaborated into the
pleural plates for further support of the legs and wings. In some of the primitive forms, lateral
movable lobes have developed from the coxapodite. Where the lobes occur on the ental margin of the coxapodite
they are identified as endites; those on the ectal
surface are exites.
Progressive evolution of a walking leg may have resulted in further
segmentation and the provision of these segments with muscles for their
articulation. In the present‑day
insect leg these segments are identified from proximal to distal as the trochanter, femur, tibia, from
one to five tarsi, and a pretarsus.
It was assumed that the appendages
of the hypothetical gnathocephalon of a prototype evolved into the
mandibulate mouthparts of a chewing insect.
A comparison of the segments and musculature of a typical maxilla with
that of a leg provides a reasonably plausible explanation for this
theory. The cardo is the
articulating segment of the maxilla and appears to be comparable with the
subcoxa of a primitive leg.
Articulating with the cardo is the stipes which seems to be homologous
with the coxa. Both coxa and
stipes bear a long, segmented telopodite.
The telopodite of the maxilla is an undifferentiated maxillary palp,
while that of the leg is a specialized series of segments identified as
trochanter, femur, tibia and tarsi.
Endites of the stipes evolved into the specialized lacinia and galea
of the food ingesting mechanism. An examination of the labium demonstrates
that it is a composite of two appendages.
Each half of the labium is essentially composed of the same basic
segments as the maxilla. The basal,
articulating postmentum is comparable with the cardo, the prementum bears the
same relationship as the stipes since it bears the 3‑segmented
telopodite or labial palp. The
endites glossa and paraglossa are comparable with the lacinea and galea of
the maxilla. The mandibles are
probably more highly evolved toward a simplification of structure. The terminal product of this
specialization is a fusion of the basic subcoxa and coxa, and an elimination
of the telopodite. In some insects, a
distinct sclerite or prostheca is discernible. This sclerite is probably an endite and is homologous with the
lacinia of the maxilla. Sincere appreciation is extended to Dr. Robert Dicke and Dr.
Dorothy Feir of the Department of Entomology, University of Wisconsin,
Madison, and to Dr. Donald Davis, Department of Entomology, Utah State
University for their suggestions, instruction and encouragement. |
---------------------------------------------
1/ Refer to Section IV ‑ Origin of the Principal Body Regions.
2/ Refer to Section V ‑ Composition of the Cuticula.