|
Radiometric Dating: A
Christian Perspective Dr. Roger C. Wiens http://www.asa3.org/ASA/resources/Wiens.html [A PDF version of this document is also available.]
Dr. Wiens has a PhD in
Physics, with a minor in Geology. His PhD thesis was on isotope ratios in
meteorites, including surface exposure dating. He was employed at Caltech's Division
of Geological & Planetary Sciences at the time of writing the first
edition. He is presently employed in the Space & Atmospheric Sciences
Group at the Los Alamos National Laboratory. First edition 1994; revised version
2002. Radiometric dating--the process of
determining the age of rocks from the decay of their radioactive
elements--has been in widespread use for over half a century. There are over forty
such techniques, each using a different radioactive element or a different
way of measuring them. It has become increasingly clear that these
radiometric dating techniques agree with each other and as a whole, present a
coherent picture in which the Earth was created a very long time ago. Further
evidence comes from the complete agreement between radiometric dates and
other dating methods such as counting tree rings or glacier ice core layers.
Many Christians have been led to distrust radiometric dating and are
completely unaware of the great number of laboratory measurements that have
shown these methods to be consistent. Many are also unaware that
Bible-believing Christians are among those actively involved in radiometric
dating. This paper describes in relatively
simple terms how a number of the dating techniques work, how accurately the
half-lives of the radioactive elements and the rock dates themselves are
known, and how dates are checked with one another. In the process the paper
refutes a number of misconceptions prevalent among Christians today. This
paper is available on the web via the American Scientific Affiliation and
related sites to promote greater understanding and wisdom on this issue,
particularly within the Christian community. ii TABLE OF CONTENTS Introduction Thermoluminescence Doubters
Still Try Appendix: Common
Misconceptions Regarding Radiometric Dating Techniques Arguments over the age of the Earth have
sometimes been divisive for people who regard the Bible as God's word. Even
though the Earth's age is never mentioned in the Bible, it is an issue
because those who take a strictly literal view of the early chapters of
Genesis can calculate an approximate date for the creation by adding up the
life-spans of the people mentioned in the genealogies. Assuming a strictly
literal interpretation of the week of creation, even if some of the
generations were left out of the genealogies, the Earth would be less than
ten thousand years old. Radiometric dating techniques indicate that the Earth
is thousands of times older than that--approximately four and a half billion
years old. Many Christians accept this and interpret the Genesis account in
less scientifically literal ways. However, some Christians suggest that the
geologic dating techniques are unreliable, that they are wrongly interpreted,
or that they are confusing at best. Unfortunately, much of the literature
available to Christians has been either inaccurate or difficult to
understand, so that confusion over dating techniques continues. The next few pages cover a broad
overview of radiometric dating techniques, show a few examples, and discuss
the degree to which the various dating systems agree with each other. The
goal is to promote greater understanding on this issue, particularly for the
Christian community. Many people have been led to be skeptical of dating
without knowing much about it. For example, most people don't realize that
carbon dating is only rarely used on rocks. God has called us to be
"wise as serpents" (Matt. 10:16) even in this scientific age. In
spite of this, differences still occur within the church. A disagreement over
the age of the Earth is relatively minor in the whole scope of Christianity;
it is more important to agree on the Rock of Ages than on the age of rocks.
But because God has also called us to wisdom, this issue is worthy of study. Rocks are made up of many individual
crystals, and each crystal is usually made up of at least several different
chemical elements such as iron, magnesium, silicon, etc. Most of the elements
in nature are stable and do not change. However, some elements are not
completely stable in their natural state. Some of the atoms eventually change
from one element to another by a process called radioactive decay. If there
are a lot of atoms of the original element, called the parent element, the
atoms decay to another element, called the daughter element, at a predictable
rate. The passage of time can be charted by the reduction in the number of
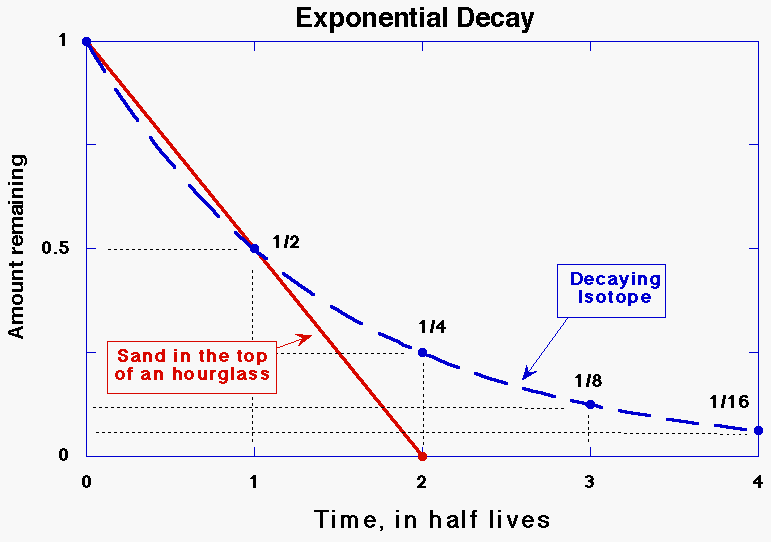
parent atoms, and the increase in the number of daughter atoms. Radiometric dating can be compared to
an hourglass. When the glass is turned over, sand runs from the top to the
bottom. Radioactive atoms are like individual grains of sand--radioactive
decays are like the falling of grains from the top to the bottom of the
glass. You cannot predict exactly when any one particular grain will get to
the bottom, but you can predict from one time to the next how long the whole
pile of sand takes to fall. Once all of the sand has fallen out of the top,
the hourglass will no longer keep time unless it is turned over again.
Similarly, when all the atoms of the radioactive element are gone, the rock
will no longer keep time (unless it receives a new batch of radioactive
atoms).
Unlike the hourglass, where the amount
of sand falling is constant right up until the end, the number of decays from
a fixed number of radioactive atoms decreases as there are fewer atoms left
to decay (see Figure 1). If it takes a certain length of time for half of the atoms
to decay, it will take the same amount of time for half of the remaining
atoms, or a fourth of the original total, to decay. In the next
interval, with only a fourth remaining, only one eighth of the original total
will decay. By the time ten of these intervals, or half-lives, has
passed, less than one thousandth of the original number of radioactive atoms
is left. The equation for the fraction of parent atoms left is very
simple. The type of equation is exponential, and is related to
equations describing other well-known phenomena such as population growth. No deviations
have yet been found from this equation for radioactive decay. Also unlike the hourglass, there is no
way to change the rate at which radioactive atoms decay in rocks. If you shake the
hourglass, twirl it, or put it in a rapidly accelerating vehicle, the time it
takes the sand to fall will change. But the radioactive atoms used in dating techniques have
been subjected to heat, cold, pressure, vacuum, acceleration, and strong
chemical reactions to the extent that would be experienced by rocks or magma
in the mantle, crust, or surface of the Earth or other planets without any
significant change in their decay rate. An hourglass will tell time correctly
only if it is completely sealed. If it has a hole allowing the sand grains to escape out
the side instead of going through the neck, it will give the wrong time
interval. Similarly, a rock that is to be dated must be sealed
against loss or addition of either the radioactive daughter or parent. If it has lost
some of the daughter element, it will give an inaccurately young age. As will be
discussed later, most dating techniques have very good ways of telling if
such a loss has occurred, in which case the date is thrown out (and so is the
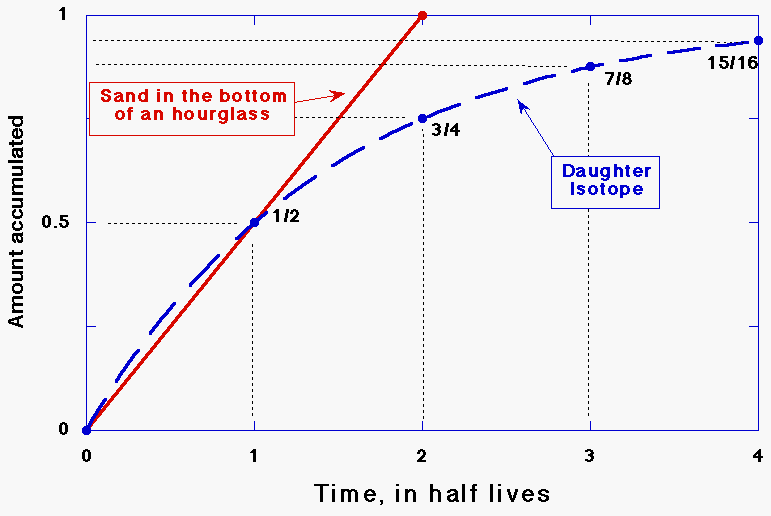
rock!). An hourglass measures how much time has
passed since it was turned over. (Actually it tells when a specific amount of time, e.g., 2
minutes, an hour, etc., has passed, so the analogy is not quite perfect.)
Radiometric dating of rocks also tells how much time has passed since some
event occurred. For igneous rocks the event is usually its cooling and
hardening from magma or lava. For some other materials, the event is the end of a
metamorphic heating event (in which the rock gets baked underground at
generally over a thousand degrees Fahrenheit), the uncovering of a surface by
the scraping action of a glacier, the chipping of a meteorite off of an asteroid,
or the length of time a plant or animal has been dead. There are now well over forty different
radiometric dating techniques, each based on a different radioactive
isotope. The term isotope subdivides elements
into groups of atoms that have the same atomic weight. For
example carbon has isotopes of weight 12, 13, and 14 times the mass of a
nucleon, referred to as carbon-12, carbon-13, or carbon-14 (abbreviated as 12C,
13C, 14C).
It is only the
carbon-14 isotope that is radioactive.
This will be
discussed further in a later section. A partial list of the parent and
daughter isotopes and the decay half-lives is given in Table I. Notice the large
range in the half-lives. Isotopes with long half-lives decay very slowly, and so are
useful for dating
correspondingly ancient events. Isotopes with
shorter half-lives cannot date very ancient events because all of the atoms of
the parent isotope would have already decayed away, like an hourglass left
sitting with all the sand at the bottom. Isotopes with relatively short half-lives are useful for
dating correspondingly shorter intervals, and can usually do so with greater
accuracy, just as you would use a stopwatch rather than a grandfather clock
to time a 100 meter dash. On the other hand, you would use a calendar, not a clock,
to record time intervals of several weeks or more. The half-lives have all been measured
directly either by using a radiation detector to count the number of atoms
decaying in a given amount of time from a known amount of the parent
material, or by measuring the ratio of daughter to parent atoms in a sample
that originally consisted completely of parent atoms. Work on
radiometric dating first started shortly after the turn of the 20th century,
but progress was relatively slow before the late forties. However, by now
we have had over fifty years to measure and re-measure the half-lives for
many of the dating techniques. Very precise counting of the decay events or the daughter
atoms can be done, so while the number of, say, rhenium-187 atoms decaying in
50 years is a very small fraction of the total, the resulting osmium-187
atoms can be very precisely counted. For example, recall that only one gram of material
contains over 1021 (1 with 21 zeros behind) atoms. Even if only
one trillionth of the atoms decay in one year, this is still millions of
decays, each of which can be counted by a radiation detector! The uncertainties on the half-lives
given in the table are all very small. All of the half-lives are known to
better than about two percent except for rhenium (5%), lutetium (3%), and
beryllium (3%). There is no evidence of any of the half-lives changing over
time. In fact, as discussed below, they have been observed to not
change at all over hundreds of thousands of years. Examples
of Dating Methods for Igneous Rocks Now let's look at how the actual dating
methods work. Igneous rocks are good candidates for dating. Recall that for
igneous rocks the event being dated is when the rock was formed from magma or
lava. When the molten material cools and hardens, the atoms are no longer
free to move about. Daughter atoms that result from radioactive decays
occurring after the rock cools are frozen in the place where they were made
within the rock. These atoms are like the sand grains accumulating in the
bottom of the hourglass. Determining the age of a rock is a two-step process.
First one needs to measure the number of daughter atoms and the number of
remaining parent atoms and calculate the ratio between them. Then the
half-life is used to calculate the time it took to produce that ratio of
parent atoms to daughter atoms. However, there is one complication. One
cannot always assume that there were no daughter atoms to begin with. It
turns out that there are some cases where one can make that assumption quite
reliably. But in most cases the initial amount of the daughter product must
be accurately determined. Most of the time one can use the different amounts
of parent and daughter present in different minerals within the rock to tell
how much daughter was originally present. Each dating mechanism deals with
this problem in its own way. Some types of dating work better in some rocks;
others are better in other rocks, depending on the rock composition and its
age. Let's examine some of the different dating mechanisms now. Potassium-Argon. Potassium is an abundant element in the Earth's crust. One isotope, potassium-40, is
radioactive and decays to two different daughter products, calcium-40 and
argon-40, by two different decay methods. This is not a problem because the production ratio of these
two daughter products is precisely known, and is always constant: 11.2%
becomes argon-40 and 88.8% becomes calcium-40. It is possible to date some rocks by the potassium-calcium
method, but this is not often done because it is hard to determine how much
calcium was initially present. Argon,
on the other hand, is a gas. Whenever
rock is melted to become magma or lava, the argon tends to escape. Once the molten material hardens,
it begins to trap the new argon produced since the hardening took place. In this way the potassium-argon
clock is clearly reset when an igneous rock is formed. In its simplest form, the geologist
simply needs to measure the relative amounts of potassium-40 and argon-40 to
date the rock. The age is
given by a relatively simple equation: t = h x ln[1 + (argon-40)/(0.112 x
(potassium-40))]/ln(2) where t is the time
in years, h is the half-life, also in years, and ln is the
natural logarithm. However, in reality there is often a
small amount of argon remaining in a rock when it hardens. This is usually trapped in the
form of very tiny air bubbles in the rock. One percent of the air we breathe is argon. Any extra argon from air bubbles
may need to be taken into account if it is significant relative to the amount
of radiogenic argon (that is, argon produced by radioactive decays). This would most likely be the case
in either young rocks that have not had time to produce much radiogenic
argon, or in rocks that are low in the parent potassium. One must have a way to determine
how much air-argon is in the rock. This
is rather easily done because air-argon has a couple of other isotopes, the
most abundant of which is argon-36. The
ratio of argon-40 to argon-36 in air is well known, at 295. Thus, if one measures argon-36 as
well as argon-40, one can calculate and subtract off the air-argon-40 to get
an accurate age. One of the best ways of showing that an
age-date is correct is to confirm it with one or more different dating
method(s). Although
potassium-argon is one of the simplest dating methods, there are still some
cases where it does not agree with other methods. When this does happen, it is usually because the gas within
bubbles in the rock is from deep underground rather than from the air. This gas can have a higher
concentration of argon-40 escaping from the melting of older rocks. This is called parentless
argon-40 because its parent potassium is not in the rock being dated, and is
also not from the air. In
these slightly unusual cases, the date given by the normal potassium-argon
method is too old. However,
scientists in the mid-1960s came up with a way around this problem, the
argon-argon method, discussed in the next section. Argon-Argon. Even though it has been around for nearly half a century,
the argon-argon method is seldom discussed by groups critical of dating
methods. This method uses
exactly the same parent and daughter isotopes as the potassium-argon method. In effect, it is a different way
of telling time from the same clock. Instead
of simply comparing the total potassium with the non-air argon in the rock,
this method has a way of telling exactly what and how much argon is directly
related to the potassium in the rock. In the argon-argon method the rock is
placed near the center of a nuclear reactor for a period of hours. A nuclear reactor emits a very
large number of neutrons, which are capable of changing a small amount of the
potassium-39 into argon-39. Argon-39
is not found in nature because it has only a 269-year half-life. (This
half-life doesn't affect the argon-argon dating method as long as the
measurements are made within about five years of the neutron dose). The rock is then heated in a
furnace to release both the argon-40 and the argon-39 (representing the
potassium) for analysis. The
heating is done at incrementally higher temperatures and at each step the
ratio of argon-40 to argon-39 is measured. If the argon-40 is from decay of potassium within the rock, it
will come out at the same temperatures as the potassium-derived argon-39 and in
a constant proportion. On
the other hand, if there is some excess argon-40 in the rock it will cause a
different ratio of argon-40 to argon-39 for some or many of the heating
steps, so the different heating steps will not agree with each other.
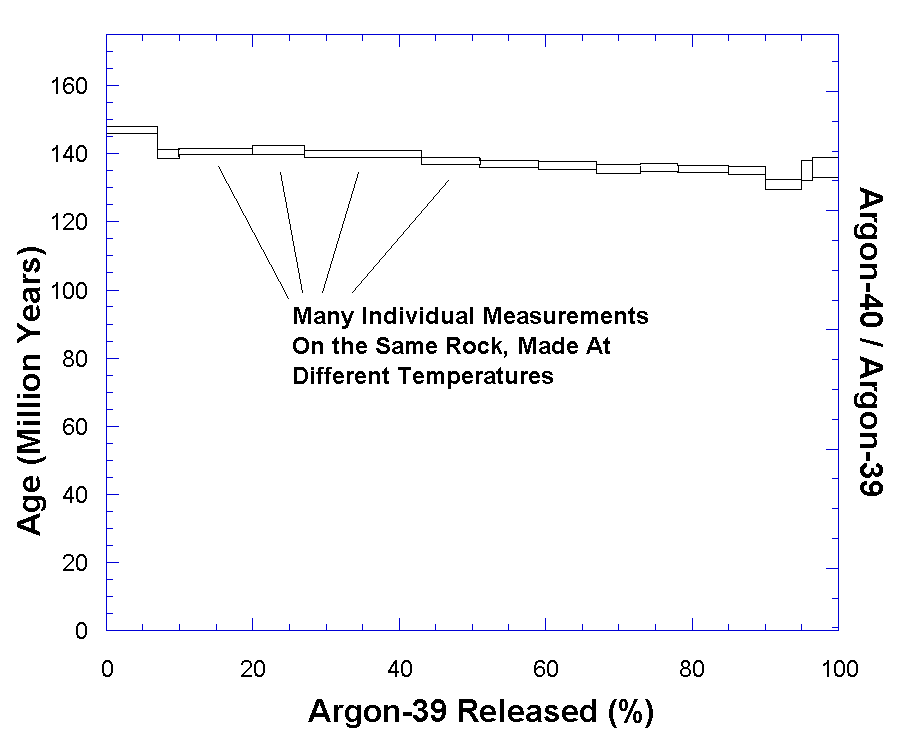
Figure 2 is an example of a good
argon-argon date. The fact that this plot is flat shows that essentially all
of the argon-40 is from decay of potassium within the rock. The potassium-40
content of the sample is found by multiplying the argon-39 by a factor based
on the neutron exposure in the reactor. When this is done, the plateau in the figure represents an
age date based on the decay of potassium-40 to argon-40. There are occasions when the
argon-argon dating method does not give an age even if there is sufficient
potassium in the sample and the rock was old enough to date. This most often
occurs if the rock experienced a high temperature (usually a thousand degrees
Fahrenheit or more) at some point since its formation. If that occurs,
some of the argon gas moves around, and the analysis does not give a smooth
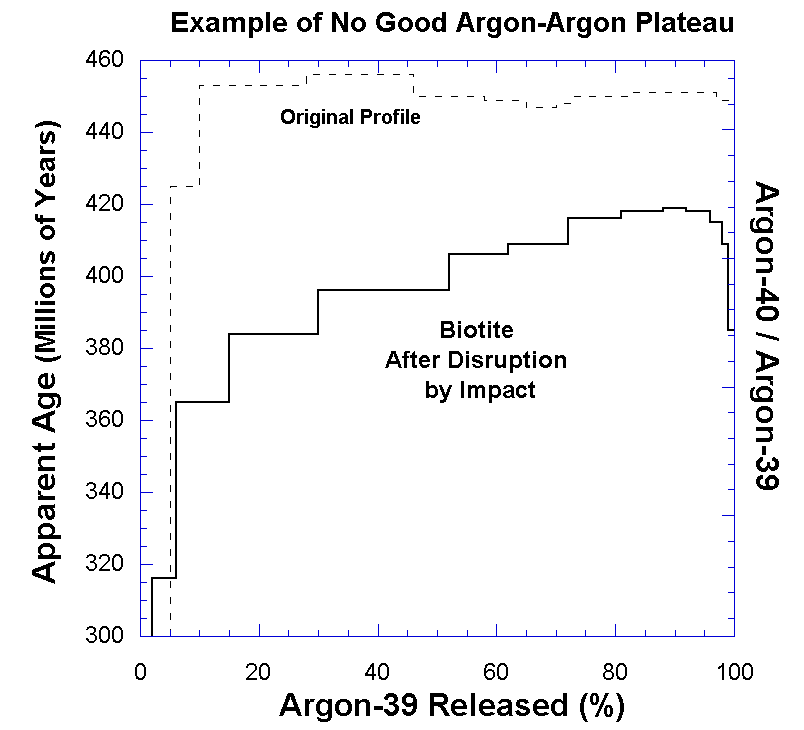
plateau across the extraction temperature steps. An example of an
argon-argon analysis that did not yield an age date is shown in Figure 3. Notice that
there is no good plateau in this plot. In some instances there will actually be two plateaus, one
representing the formation age, and another representing the time at which
the heating episode occurred. But in most cases where the system has been disturbed,
there simply is no date given. The important point to note is that, rather than giving
wrong age dates, this method simply does not give a date if the system has
been disturbed. This is also true of a number of other igneous rock dating
methods, as we will describe below.
Rubidium-Strontium. In nearly all of the dating methods, except
potassium-argon and the associated argon-argon method, there is always some
amount of the daughter product already in the rock when it cools. Using these methods is a little
like trying to tell time from an hourglass that was turned over before all of
the sand had fallen to the bottom. One
can think of ways to correct for this in an hourglass: One could make a mark
on the outside of the glass where the sand level started from and then repeat
the interval with a stopwatch in the other hand to calibrate it. Or if one is clever she or he
could examine the hourglass' shape and determine what fraction of all the
sand was at the top to start with. By
knowing how long it takes all of the sand to fall, one could determine how
long the time interval was. Similarly,
there are good ways to tell quite precisely how much of the daughter product
was already in the rock when it cooled and hardened. In the
rubidium-strontium method, rubidium-87 decays with a half-life of 48.8
billion years to strontium-87. Strontium has several other isotopes that are
stable and do not decay. The ratio of strontium-87 to one of the other stable
isotopes, say strontium-86, increases over time as more rubidium-87 turns to
strontium-87. But when the rock first cools, all parts of the rock have the
same strontium-87/strontium-86 ratio because the isotopes were mixed in the
magma. At the same time, some of the minerals in the rock have a higher
rubidium/strontium ratio than others. Rubidium has a larger atomic diameter
than strontium, so rubidium does not fit into the crystal structure of some
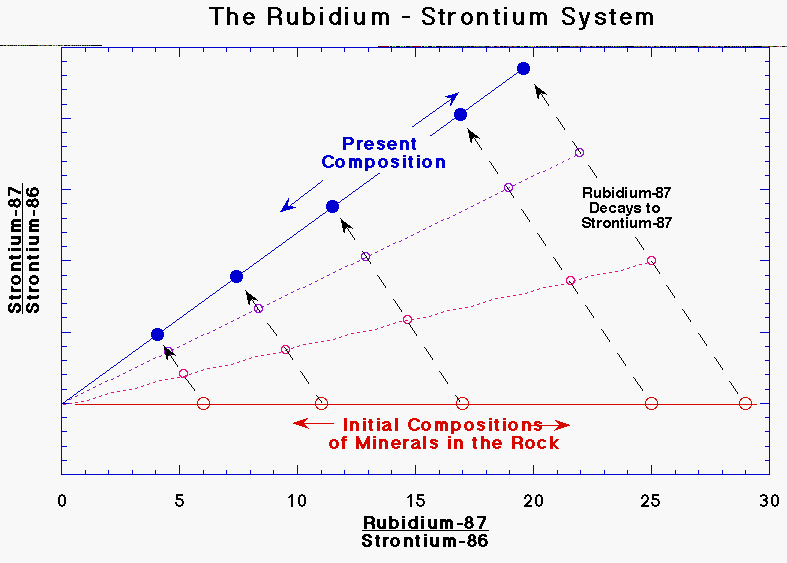
minerals as well as others. Figure 4 is an important type of plot
used in rubidium-strontium dating. It shows the strontium-87/strontium-86
ratio on the vertical axis and the
Figure
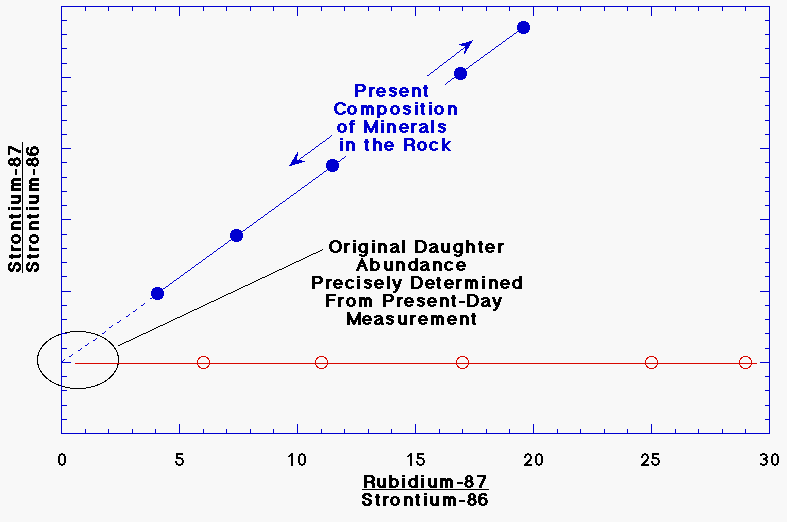
5. The original amount of the daughter
strontium-87 can be precisely determined from the present-day composition by
extending the line through the data points back to rubidium-87 = 0. This
works because if there were no rubidium-87 in the sample, the strontium
composition would not change. The slope of the line is used to determine the
age of the sample. rubidium-87/strontium-86 ratio on the
horizontal axis, that is, it plots a ratio of the daughter isotope against a
ratio of the parent isotope. At first, all the minerals lie along a
horizontal line of constant strontium-87/strontium-86 ratio but with varying
rubidium/strontium. As the rock starts to age, rubidium gets converted to
strontium. The amount of strontium added to each mineral is proportional to
the amount of rubidium present. This change is shown by the dashed arrows,
the lengths of which are proportional to the rubidium/strontium ratio. The
dashed arrows are slanted because the rubidium/strontium ratio is decreasing
in proportion to the increase in strontium-87/strontium-86. The solid line
drawn through the samples will thus progressively rotate from the horizontal
to steeper and steeper slopes. All lines drawn through the data points
at any later time will intersect the horizontal line (constant
strontium-87/strontium-86 ratio) at the same point in the lower left-hand
corner. This point, where rubidium-87/strontium-86 = 0 tells the original
strontium-87/strontium-86 ratio. From that we can determine the original
daughter strontium-87 in each mineral, which is just what we need to know to
determine the correct age. It also turns out that the slope of the
line is proportional to the age of the rock. The older the rock, the steeper
the line will be. If the slope of the line is m and the half-life is h,
the age t (in years) is given by the equation t = h x ln(m+1)/ln(2) For a system with a very long half-life
like rubidium-strontium, the actual numerical value of the slope will always
be quite small. To give an example for the above equation, if the slope of a
line in a plot similar to Fig. 4 is m = 0.05110 (strontium isotope ratios are
usually measured very accurately--to about one part in ten thousand), we can substitute in the half-life
(48.8 billion years) and solve as follows: t = (48.8) x
ln(1.05110)/ln(2) so t = 3.51 billion years. Several things can on rare occasions
cause problems for the rubidium-strontium dating method. One possible
source of problems is if a rock contains some minerals that are older than
the main part of the rock. This can happen when magma inside the Earth picks up
unmelted minerals from the surrounding rock as the magma moves through a
magma chamber. Usually a good geologist can distinguish these
"xenoliths" from the younger minerals around them. If he or she
does happen to use them for dating the rock, the points represented by these
minerals will lie off the line made by the rest of the points. Another
difficulty can arise if a rock has undergone metamorphism, that is, if the
rock got very hot, but not hot enough to completely re-melt the rock. In these cases,
the dates look confused, and do not lie along a line. Some of the
minerals may have completely melted, while others did not melt at all, so
some minerals try to give the igneous age while other minerals try to give
the metamorphic age. In these cases there will not be a straight line, and no
date is determined. In a few very rare instances the
rubidium-strontium method has given straight lines that give wrong ages. This can happen
when the rock being dated was formed from magma that was not well mixed, and
which had two distinct batches of rubidium and strontium. One magma batch
had rubidium and strontium compositions near the upper end of a line (such as
in Fig. 4), and one batch had compositions near the lower end of the line. In this case,
the minerals all got a mixture of these two
batches, and their resulting composition ended up near a line between the two
batches. This is called a two-component mixing line. It is a very
rare occurrence in these dating mechanisms, but at least thirty cases have
been documented among the tens of thousands of rubidium-strontium dates made. If a two-component mixture is suspected, a
second dating method must be used to confirm or disprove the
rubidium-strontium date. The agreement of several dating methods is the best
fail-safe way of dating rocks. The
Samarium-Neodymium, Lutetium-Hafnium, and Rhenium-Osmium
Methods. All of these
methods work very similarly to the rubidium-strontium method. They all use
three-isotope diagrams similar to Figure 4 to determine the age. The
samarium-neodymium method is the most-often used of these three. It uses the
decay of samarium-147 to neodymium-143, which has a half-life of 105 billion
years. The ratio of the daughter isotope, neodymium-143, to another neodymium
isotope, neodymium-144, is plotted against the ratio of the parent,
samarium-147, to neodymium-144. If different minerals from the same rock plot
along a line, the slope is determined, and the age is given by the same
equation as above. The samarium-neodymium method may be preferred for rocks
that have very little potassium and rubidium, for which the potassium-argon,
argon-argon, and rubidium-strontium methods might be difficult. The
samarium-neodymium method has also been shown to be more resistant to being
disturbed or re-set by metamorphic heating events, so for some metamorphosed
rocks the samarium-neodymium method is preferred. For a rock of the same age,
the slope on the neodymium-samarium plots will be less than on a
rubidium-strontium plot because the half-life is longer. However, these
isotope ratios are usually measured to extreme accuracy--several parts in ten
thousand--so accurate dates can be obtained even for ages less than one
fiftieth of a half-life, and with correspondingly small slopes. The lutetium-hafnium method uses the 38
billion year half-life of lutetium-176 decaying to hafnium-176. This dating
system is similar in many ways to samarium-neodymium, as the elements tend to
be concentrated in the same types of minerals. Since samarium-neodymium
dating is somewhat easier, the lutetium-hafnium method is used less often. The rhenium-osmium method takes
advantage of the fact that the osmium concentration in most rocks and
minerals is very low, so a small amount of the parent rhenium-187 can produce
a significant change in the osmium isotope ratio. The half-life for this
radioactive decay is 42 billion years. The non-radiogenic stable isotopes,
osmium-186 or -188, are used as the denominator in the ratios on the
three-isotope plots. This method has been useful for dating iron meteorites,
and is now enjoying greater use for dating Earth rocks due to development of
easier rhenium and osmium isotope measurement techniques. Uranium-Lead and related techniques. The uranium-lead method is the longest-used dating method. It was first used in 1907, about a
century ago. The uranium-lead
system is more complicated than other parent-daughter systems; it is actually
several dating methods put together. Natural
uranium consists primarily of two isotopes, U-235 and U-238, and these
isotopes decay with different half-lives to produce lead-207 and lead-206,
respectively. In addition,
lead-208 is produced by thorium-232. Only
one isotope of lead, lead-204, is not radiogenic. The uranium-lead system has an interesting complication: none
of the lead isotopes is produced directly from the uranium and thorium. Each decays through a series of
relatively short-lived radioactive elements that each decay to a lighter
element, finally ending up at lead. Since these half-lives are so short
compared to U-238, U-235, and thorium-232, they generally do not affect the
overall dating scheme. The
result is that one can obtain three independent estimates of the age of a
rock by measuring the lead isotopes and their parent isotopes. Long-term dating based on the
U-238, U-235, and thorium-232 will be discussed briefly here; dating based on
some of the shorter-lived intermediate isotopes is discussed later. The uranium-lead system in its simpler
forms, using U-238, U-235, and thorium-232, has proved to be less reliable
than many of the other dating systems.
This is because both uranium and lead are less easily retained in many
of the minerals in which they are found. Yet the fact that there are three dating systems all in one
allows scientists to easily determine whether the system has been disturbed
or not. Using slightly more
complicated mathematics, different combinations of the lead isotopes and
parent isotopes can be plotted in such a way as to minimize the effects of lead loss. One of these techniques is called
the lead-lead technique because it determines the ages from the lead isotopes
alone. Some of these
techniques allow scientists to chart at what points in time metamorphic
heating events have occurred, which is also of significant interest to
geologists.
We now turn our attention to what the
dating systems tell us about the age of the Earth.
The most obvious constraint is
the age of the oldest rocks. These have been dated at up to about four billion years. But actually
only a very small portion of the Earth's rocks are that old. From satellite data and other measurements we know that
the Earth's surface is constantly rearranging itself little by little as
Earthquakes occur. Such rearranging cannot occur without some of the Earth's
surface disappearing under other parts of the Earth's surface, re-melting
some of the rock. So it appears that none of the rocks have survived from
the creation of the Earth without undergoing remelting, metamorphism, or
erosion, and all we can say--from this line of evidence--is that the Earth
appears to be at least as old as the four billion year old rocks. When scientists began systematically
dating meteorites they learned a very interesting thing: nearly all of the
meteorites had practically identical ages, at 4.56 billion years. These meteorites
are chips off the asteroids. When the asteroids were formed in space, they cooled
relatively quickly (some of them may never have gotten very warm), so all of
their rocks were formed within a few million years. The asteroids'
rocks have not been remelted ever since, so the ages have generally not been
disturbed. Meteorites that show evidence of being from the largest
asteroids have slightly younger ages. The moon is larger than the largest asteroid. Most of the
rocks we have from the moon do not exceed 4.1 billion years. The samples
thought to be the oldest are highly pulverized and difficult to date, though
there are a few dates extending all the way to 4.4 to 4.5 billion years. Most scientists
think that all the bodies in the solar system were created at about the same
time. Evidence
from the uranium, thorium, and lead isotopes links the Earth's age with that
of the meteorites. This would make the Earth 4.5-4.6 billion years old. Extinct Radionuclides: The
Hourglasses That Ran Out
There is another way to determine the
age of the Earth. If we see an hourglass whose sand has run out, we know
that it was turned over longer ago than the time interval it measures. Similarly, if we
find that a radioactive parent was once abundant but has since run out, we
know that it too was set longer ago than the time interval it measures. There
are in fact many, many more parent isotopes than those listed in Table 1.
However, most of them are no longer found naturally on Earth--they have run
out. Their
half-lives range down to times shorter than we can measure. Every single
element has radioisotopes that no longer exist on Earth! Many people are familiar with a chart
of the elements (Fig. 6). Nuclear chemists and geologists use a different kind of
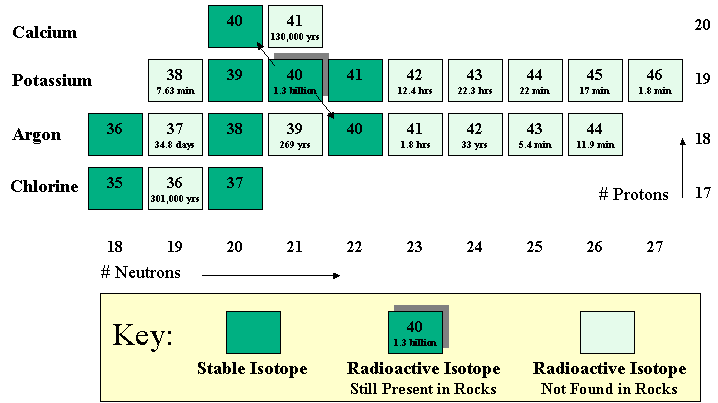
figure to show all of the isotopes. It is called a chart of the nuclides. Figure 7 shows a
portion of this chart. It is basically a plot of the number of protons vs. the
number of neutrons for various isotopes. Recall that an element is defined by
how many protons it has. Each element can have a number of different
isotopes, that is,
atoms with different numbers of
neutrons. So each element occupies a single row, while different isotopes of
that element lie in different columns. For potassium found in nature, the
total neutrons plus protons can add up to 39, 40, or 41. Potassium-39 and -41
are stable, but potassium-40 is unstable, giving us the dating methods
discussed above. Besides the stable potassium isotopes and potassium-40, it
is possible to produce a number of other potassium isotopes, but, as shown by
the half-lives of these isotopes off to the side, they decay away rather quickly. Now, if we look at which radioisotopes
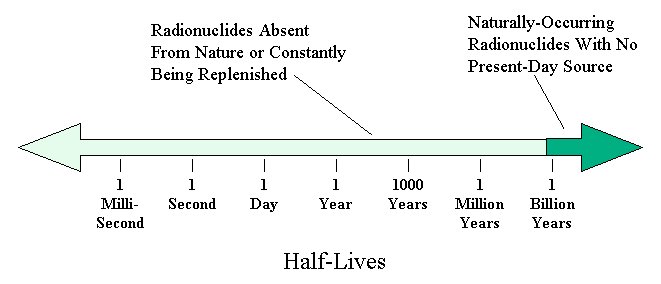
still exist and which do not, we find a very interesting fact. Nearly
all isotopes with half-lives shorter than half a billion years are no longer
in existence. For example, although most rocks contain significant amounts of
Calcium, the isotope Calcium-41 (half-life 130,000 years does not exist just
as potassium-38, -42, -43, etc. do not (Fig. 7). Just about the only
radioisotopes found naturally are those with very long half-lives of close to
a billion years or longer, as illustrated in the time line in Fig. 8. The
only isotopes present with shorter half-lives are those that have a source
constantly replenishing them. Chlorine-36 (shown in Fig. 7) is one such
"cosmogenic" isotope, as we are about to discuss below. In a number
of cases there is
evidence, particularly in meteorites,
that shorter-lived isotopes existed at some point in the past, but have since
become extinct. Some of these isotopes and their half-lives are given in
Table II. This is conclusive evidence that the solar system was created
longer ago than the span of these half lives! On the other hand, the
existence in nature of parent isotopes with half lives around a billion years
and longer is strong evidence that the Earth was created not longer ago than
several billion years. The Earth is old enough that radioactive isotopes with
half-lives less than half a billion years decayed away, but not so old that
radioactive isotopes with longer half-lives are gone. This is just like
finding hourglasses measuring a long time interval still going, while
hourglasses measuring shorter intervals have run out. Cosmogenic Radionuclides: Carbon-14,
Beryllium-10, Chlorine-36
The last 5 radiometric systems listed
up in Table I have far shorter half-lives than all the rest. Unlike the
radioactive isotopes discussed above, these isotopes are constantly being replenished
in small amounts in one of two ways. The bottom two entries, uranium-234 and
thorium-230, are replenished as the long-lived uranium-238 atoms decay. These
will be discussed in the next section. The other three, Carbon-14,
beryllium-10, and chlorine-36 are produced by cosmic rays--high energy
particles and photons in space--as they hit the Earth's upper atmosphere.
Very small amounts of each of these isotopes are present in the air we
breathe and the water we drink. As a result, living things, both plants and
animals, ingest very small amounts of carbon-14, and lake and sea sediments
take up small amounts of beryllium-10 and chlorine-36. The cosmogenic dating clocks work
somewhat differently than the others. Carbon-14 in particular is used to date
material such as bones, wood, cloth, paper, and other dead tissue from either
plants or animals. To a rough approximation, the ratio of carbon-14 to the
stable isotopes, carbon-12 and carbon-13, is relatively constant in the
atmosphere and living organisms, and has been well calibrated. Once a living
thing dies, it no longer takes in carbon from food or air, and the amount of
carbon-14 starts to drop with time. How far the carbon-14/carbon-12 ratio has
dropped indicates how old the sample is. Since the half-life of carbon-14 is
less than 6,000 years, it can only be used for dating material less than
about 45,000 years old. Dinosaur bones do not have carbon-14 (unless
contaminated), as the dinosaurs became extinct over 60 million years ago. But
some other animals that are now extinct, such as North American mammoths, can
be dated by carbon-14. Also, some materials from prehistoric times, as well
as Biblical events, can be dated by carbon-14. The carbon-14 dates have been carefully
cross-checked with non-radiometric age indicators. For example growth rings
in trees, if counted carefully, are a reliable way to determine the age of a
tree. Each growth ring only collects carbon from the air and nutrients during
the year it is made. To calibrate carbon-14, one can analyze carbon from the
center several rings of a tree, and then count the rings inward from the
living portion to determine the actual age. This has been done for the
"Methuselah of trees", the bristlecone pine trees, which grow very slowly
and live up to 6,000 years. Scientists have extended this calibration even
further. These trees grow in a very dry region near the California-Nevada
border. Dead trees in this dry climate take many thousands Tree rings do
not provide continuous chronologies beyond 11,800 years ago because a rather
abrupt change in climate took place at that time, which was the end of the
last ice age. During the ice age, long-lived trees grew in different areas
than they do now. There are many indicators, some to be mentioned below, that
show exactly how the climate changed at the end of the last ice age. It is
difficult to find continuous tree ring records through this period of rapid
climate change. Dendrochronology will probably eventually find reliable tree
records that bridge this time period, but in the meantime, the carbon-14 ages
have been calibrated farther back in time by other means. Calibration of carbon-14 back to almost
50,000 years ago has been done in several ways. One way is to find yearly
layers that are produced over longer periods of time than tree rings. In some
lakes or bays where underwater sedimentation occurs at a relatively rapid
rate, the sediments have seasonal patterns, so each year produces a distinct
layer. Such sediment layers are called "varves", and are described
in more detail below. Varve layers can be counted just like tree rings. If
layers contain dead plant material, they can be used to calibrate the
carbon-14 ages. Another way to calibrate carbon-14
farther back in time is to find recently-formed carbonate deposits and
cross-calibrate the carbon-14 in them with another short-lived radioactive
isotope. Where do we find recently-formed carbonate deposits? If you have
ever taken a tour of a cave and seen water dripping from stalactites on the
ceiling to stalagmites on the floor of the cave, you have seen carbonate
deposits being formed. Since most cave formations have formed relatively
recently, formations such as stalactites and stalagmites have been quite
useful in cross-calibrating the carbon-14 record. What does one find in the calibration
of carbon-14 against actual ages? If one predicts a carbon-14 age assuming
that the ratio of carbon-14 to carbon-12 in the air has stayed constant,
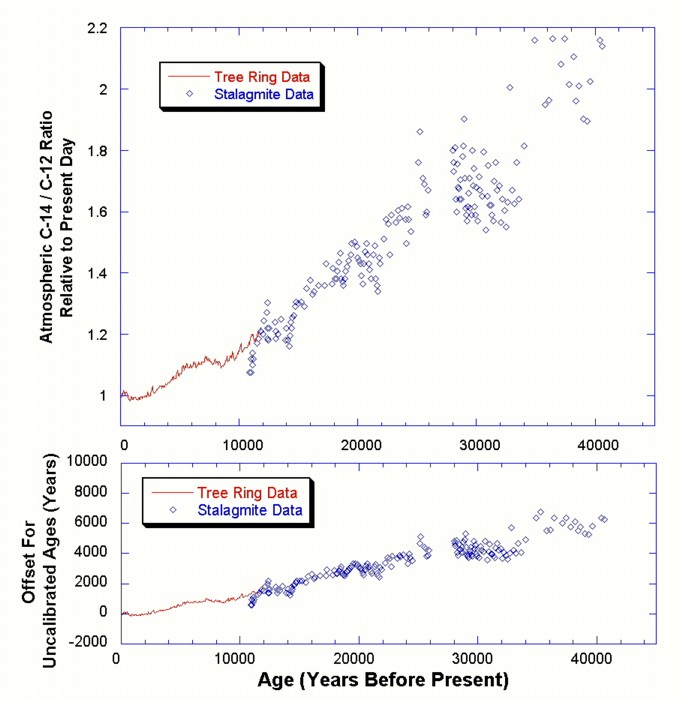
there is a slight error because this ratio has changed slightly. Figure 9
shows that the carbon-14 fraction in the air has decreased over the last
40,000 years by about a factor of two. This is attributed to a strengthening
of the Earth's magnetic field during this time. A stronger magnetic field
shields the upper atmosphere better from charged cosmic rays, resulting in
less carbon-14 production now than in the past. (Changes in the Earth's
magnetic field are well documented. Complete reversals of the north and south
magnetic poles have occurred many times over geologic history.) A small
amount of data beyond 40,000 years (not shown in Fig. 9) suggests that this
trend reversed between 40,000 and 50,000 years, with lower carbon-14 to
carbon-12 ratios farther back in time, but these data need to be confirmed. What change does this have on
uncalibrated carbon-14 ages? The bottom panel of Figure 9 shows the amount
of offset in the uncalibrated ages. The
offset is generally less than 1500 years over the last 10,000 years, but
grows to about 6,000 years at 40,000 years before present. Uncalibrated
radiocarbon ages underestimate the actual ages. Note that a factor of
two difference in the atmospheric carbon-14 ratio, as shown in the top panel
of Figure 9, does not translate to a factor of two offset in the age. Rather,
the offset is equal to one half-life, or 5,700 years for carbon-14. This is
only about 15% of the age of samples at 40,000 years. The initial portion of
the calibration curve in Figure 9 has been widely available and well accepted
for some time, so reported radiocarbon dates for ages up to 11,800 years
generally give the calibrated ages unless otherwise stated. The calibration
curve over the portions extending to 40,000 years is relatively recent, but
should become widely adopted as well. Radiometric Dating of Geologically Young Samples (<100,000
Years) It is sometimes possible to date
geologically young samples using some of the long-lived methods described
above. These methods may work on young samples, for example, if there is a
relatively high concentration of the parent isotope in the sample. In that
case, sufficient daughter isotope amounts are produced in a relatively short
time. As an example, an article in Science magazine (vol. 277, pp.
1279-1280, 1997) reports the agreement between the argon-argon method and the
actual known age of lava from the famous eruption of Vesuvius in Italy in 79
A.D. There are other ways to date some
geologically young samples. Besides the cosmogenic radionuclides discussed
above, there is one other class of short-lived radionuclides on Earth. These
are ones produced by decay of the long-lived radionuclides given in the upper
part of Table 1. As mentioned in the Uranium-Lead section, uranium does not
decay immediately to a stable isotope, but decays through a number of shorter-lived
radioisotopes until it ends up as lead. While the uranium-lead system can
measure intervals in the millions of years generally without problems from
the intermediate isotopes, those intermediate isotopes with the longest
half-lives span long enough time intervals for dating events less than
several hundred thousand years ago. (Note that these intervals are well under
a tenth of a percent of the half-lives of the long-lived parent uranium and
thorium isotopes discussed earlier.) Two of the most frequently-used of these
"uranium-series" systems are uranium-234 and thorium-230. These are
listed as the last two entries in Table 1, and are illustrated in Figure 10.
Like carbon-14, the shorter-lived
uranium-series isotopes are constantly being replenished, in this case, by
decaying uranium-238 supplied to the Earth during its original creation.
Following the example of carbon-14, you may guess that one way to use these
isotopes for dating is to remove them from their source of replenishment.
This starts the dating clock. In carbon-14 this happens when a living thing
(like a tree) dies and no longer takes in carbon-14-laden CO2. For
the shorter-lived uranium-series radionuclides, there needs to be a physical
removal from uranium. The chemistry of uranium and thorium are such that they
are in fact easily removed from each other. Uranium tends to stay dissolved
in water, but thorium is insoluble in water. So a number of applications of
the thorium-230 method are based on this chemical partition between uranium
and thorium. Sediments at the bottom of the ocean
have very little uranium relative to the thorium. Because of this, the
uranium, and its contribution to the thorium abundance, can in many cases be
ignored in sediments. Thorium-230 then behaves similarly to the long-lived
parent isotopes we discussed earlier. It acts like a simple parent-daughter
system, and it can be used to date sediments. On the other hand, calcium carbonates
produced biologically (such as in corals, shells, teeth, and bones) take in
small amounts of uranium, but essentially no thorium (because of its much
lower concentrations in the water). This allows the dating of these materials
by their lack of thorium. A brand-new coral reef will have essentially
no thorium-230. As it ages, some of its uranium decays to thorium-230. While
the thorium-230 itself is radioactive, this can be corrected for. The equations
are more complex than for the simple systems described earlier, but the
uranium-234 / thorium-230 method has been used to date corals now for several
decades. Comparison of uranium-234 ages with ages obtained by counting annual
growth bands of corals proves that the technique is highly accurate when properly used
(Edwards et al., Earth Planet. Sci. Lett. 90, 371, 1988). The
method has also been used to date stalactites and stalagmites from caves,
already mentioned in connection with long-term calibration of the radiocarbon
method. In fact, tens of thousands of uranium-series dates have been
performed on cave formations around the world.
Non-Radiometric Dating Methods for the
Past 100,000 Years We will digress briefly from
radiometric dating to talk about other dating techniques. It is important to
understand that a very large number of accurate dates covering the past
100,000 years has been obtained from many other methods besides radiometric
dating. We have already mentioned dendrochronology (tree ring dating) above.
Dendrochronology is only the tip of the iceberg in terms of non-radiometric
dating methods. Here we will look briefly at some other non-radiometric
dating techniques. Ice Cores.
One of the best ways to measure
farther back in time than tree rings is by using the seasonal variations in
polar ice from Greenland and Antarctica. There are a number of differences
between snow layers made in winter and those made in spring, summer, and
fall. These seasonal layers can be counted just like tree rings. The seasonal
differences consist of a) visual differences caused by increased bubbles and
larger crystal size from summer ice compared to winter ice, b) dust layers
deposited each summer, c) nitric acid concentrations, measured by electrical
conductivity of the ice, d) chemistry of contaminants in the ice, and e)
seasonal variations in the relative amounts of heavy hydrogen (deuterium) and
heavy oxygen (oxygen-18) in the ice. These isotope ratios are sensitive to
the temperature at the time they fell as snow from the clouds. The heavy
isotope is lower in abundance during the colder winter snows than it is in
snow falling in spring and summer. So the yearly layers of ice can be tracked
by each of these five different indicators, similar to growth rings on trees.
The different types of layers are summarized in Table III. Ice cores are obtained by drilling very
deep holes in the ice caps on Greenland and Antarctica with specialized
drilling rigs. As the rigs drill down, the drill bits cut around a portion of
the ice, capturing a long undisturbed "core" in the process. These
cores are carefully brought back to the surface in sections, where they are
catalogued, and taken to research laboratories under refrigeration. A very
large amount of work has been done on several deep ice cores up to 9,000 feet
in depth. Several hundred thousand measurements are sometimes made for
a single technique on a single ice core. A continuous count of layers exists
back as far as 160,000 years. In addition to yearly layering, individual
strong events (such as large-scale volcanic eruptions) can be observed and
correlated between ice cores. A number of historical eruptions as far back as
Vesuvius nearly 2,000 years ago serve as benchmarks with which to determine
the accuracy of the yearly layers as far down as around 500 meters. As one
goes further down in the ice core, the ice becomes more compacted than near
the surface, and individual yearly layers are slightly more difficult to
observe. For this reason, there is some uncertainty as one goes back towards
100,000 years. Ages of 40,000 years or less are estimated to be off by 2% at
most. Ages of 60,000 years may be off by up to 10%, and the uncertainty rises
to 20% for ages of 110,000 years based on direct counting of layers (D. Meese
et al., J. Geophys. Res. 102, 26,411, 1997). Recently, absolute ages
have been determined to 75,000 years for at least one location using
cosmogenic radionuclides chlorine-36 and beryllium-10 (G. Wagner et al., Earth
Planet. Sci. Lett. 193, 515, 2001). These agree with the ice flow
models and the yearly layer counts. Note that there is no indication anywhere
that these ice caps were ever covered by a large body of water, as some
people with young-Earth views would expect. Table III. Polar ice core layers, counting back yearly layers,
consist of the following:
Varves. Another
layering technique uses seasonal variations in sedimentary layers deposited
underwater. The two requirements for varves to be useful in dating are 1) that
sediments vary in character through the seasons to produce a visible yearly
pattern, and 2) that the lake bottom not be disturbed after the layers are
deposited. These conditions are most often met in small, relatively deep
lakes at mid to high latitudes. Shallower lakes typically experience an
overturn in which the warmer water sinks to the bottom as winter approaches,
but deeper lakes can have persistently thermally stratified
(temperature-layered) water masses, leading to less turbulence, and better
conditions for varve layers. Varves can be harvested by coring drills,
somewhat similar to the harvesting of ice cores discussed above. Overall,
many hundreds of lakes have been studied for their varve patterns. Each
yearly varve layer consists of a) mineral matter brought in by swollen
streams in the spring. b) This gradually gives way to organic particulate
matter such as plant fibers, algae, and pollen with fine-grained
mineral matter, consistent with summer and fall
deposition. c) With winter ice covering the lake, fine-grained organic
matter provides the final part of the yearly layer. Regular sequences of
varves have been measured going back to about 35,000 years. The thicknesses
of the layers and the types of material in them tells a lot about the climate
of the time when the layers were deposited. For example, pollens entrained in
the layers can tell what types of plants were growing nearby at a particular
time. Other annual layering methods. Besides
tree rings, ice cores, and sediment varves, there are other processes that
result in yearly layers that can be counted to determine an age. Annual
layering in coral reefs can be used to date sections of coral. Coral
generally grows at rates of around 1 cm per year, and these layers are easily
visible. As was mentioned in the uranium-series section, the counting of
annual coral layers was used to verify the accuracy of the thorium-230
method. Thermoluminescence. There
is a way of dating minerals and pottery that does not rely directly on
half-lives. Thermoluminescence dating, or TL dating, uses the fact that
radioactive decays cause some electrons in a material to end up stuck in
higher-energy orbits. The number of electrons in higher-energy orbits
accumulates as a material experiences more natural radioactivity over time.
If the material is heated, these electrons can fall back to their original
orbits, emitting a very tiny amount of light. If the heating occurs in a
laboratory furnace equipped with a very sensitive light detector, this light
can be recorded. (The term comes from putting together thermo, meaning
heat, and luminescence, meaning to emit light). By comparison of the
amount of light emitted with the natural radioactivity rate the sample
experienced, the age of the sample can be determined. TL dating can generally
be used on samples less than half a million years old. Related techniques
include optically stimulated luminescence (OSL), and infrared stimulated
luminescence (IRSL). TL dating and its related techniques have been cross
calibrated with samples of known historical age and with radiocarbon and
thorium dating. While TL dating does not usually pinpoint the age with as
great an accuracy as these other conventional radiometric dating, it is most
useful for applications such as pottery or fine-grained volcanic dust, where
other dating methods do not work as well. Electron spin resonance (ESR). Also called electron paramagnetic resonance, ESR dating
also relies on the changes in electron orbits and spins caused by
radioactivity over time. However, ESR dating can be used over longer time
periods, up to two million years, and works best on carbonates, such as in
coral reefs and cave deposits. It has also seen extensive use in dating tooth
enamel. Cosmic-ray exposure dating. This
dating method relies on measuring certain isotopes produced by cosmic ray
impacts on exposed rock surfaces. Because cosmic rays constantly bombard
meteorites flying through space, this method has long been used to date the '
flight time' of meteorites--that is the time from when they were chipped off
a larger body (like an asteroid) to the time they land on Earth. The cosmic
rays produce small amounts of naturally-rare isotopes such as neon-21 and
helium-3, which can be measured in the laboratory. The cosmic-ray exposure
ages of meteorites are usually around 10 million years, but can be up to a
billion years for some iron meteorites. In the last fifteen years, people
have also used cosmic ray exposure ages to date rock surfaces on the Earth.
This is much more complicated because the Earth's magnetic field and
atmosphere shield us from most of the cosmic rays. Cosmic ray exposure
calibrations must take into account the elevation above sea level because
the atmospheric shielding varies with elevation, and must also take into
account latitude, as the magnetic shielding varies from the equator to the
poles. Nevertheless, terrestrial cosmic-ray exposure dating has been shown to
be useful in many cases. Can We Really Believe the Dating Systems? We have covered a lot of convincing
evidence that the Earth was created a very long time ago. The agreement of
many different dating methods, both radiometric and non-radiometric, over
hundreds of thousands of samples, is very convincing. Yet, some
Christians question whether we can believe something so far back in the past.
My answer is that it is similar to believing in other things of the past. It
only differs in degree. Why do you believe Abraham Lincoln ever lived?
Because it would take an extremely elaborate scheme to make up his existence,
including forgeries, fake photos, and many other things, and besides, there
is no good reason to simply have made him up. Well, the situation is very
similar for the dating of rocks, only we have rock records rather than
historical records. Consider the following:
The last three points deserve more
attention. Some Christians have argued that something may be slowly changing
with time so all the ages look older than they really are. The only two
quantities in the exponent of a decay rate equation are the half-life and the
time. So for ages to appear longer than actual, all the half-lives would have
to be changing in sync with each other. One could consider that time itself
was changing if that happened (remember that our clocks are now standardized
to atomic clocks!). And such a thing would have to have occurred without our
detection in the last hundred years, which is already 5% of the way back to
the time of Christ. Beyond this, scientists have now used a
"time machine" to prove that the half-lives of radioactive species
were the same millions of years ago. This time machine does not allow people
to actually go back in time, but it does allow scientists to observe ancient
events from a long way away. The time machine is called the telescope.
Because God's universe is so large, images from distant events take a long
time to get to us. Telescopes allow us to see supernovae (exploding stars) at
distances so vast that the pictures take hundreds of thousands to millions of
years to arrive at the Earth. So the events we see today actually occurred
hundreds of thousands to millions of years ago. And what do we see when we
look back in time? Much of the light following a supernova blast is powered
by newly created radioactive parents. So we observe radiometric decay in the
supernova light. The half-lives of decays occurring hundreds of thousands of
years ago are thus carefully recorded! These half-lives completely agree with
the half-lives measured from decays occurring today. We must conclude that
all evidence points towards unchanging radioactive half-lives. Some individuals have suggested that
the speed of light must have been different in the past, and that the
starlight has not really taken so long to reach us. However, the astronomical
evidence mentioned above also suggests that the speed of light has not changed,
or else we would see a significant apparent change in the half-lives of these
ancient radioactive decays. Some doubters have tried to dismiss
geologic dating with a sleight of hand by saying that no rocks are completely
closed systems (that is, that no rocks are so isolated from their
surroundings that they have not lost or gained some of the isotopes used for
dating). Speaking from an extreme technical viewpoint this might be
true--perhaps 1 atom out of 1,000,000,000,000 of a certain isotope has leaked
out of nearly all rocks, but such a change would make an immeasurably small
change in the result. The real question to ask is, "is the rock
sufficiently close to a closed system that the results will be same as a
really closed system?" Since the early 1960s many books have been
written on this subject. These books detail experiments showing, for a given
dating system, which minerals work all of the time, which minerals work under
some certain conditions, and which minerals are likely to lose atoms and give
incorrect results. Understanding these conditions is part of the science of
geology. Geologists are careful to use the most reliable methods whenever
possible, and as discussed above, to test for agreement between different
methods. Some people have tried to defend a
young Earth position by saying that the half-lives of radionuclides can in
fact be changed, and that this can be done by certain little-understood
particles such as neutrinos, muons, or cosmic rays. This is stretching it.
While certain particles can cause nuclear changes, they do not change the
half-lives. The nuclear changes are well understood and are nearly always
very minor in rocks. In fact the main nuclear changes in rocks are the very
radioactive decays we are talking about. There are only three quite technical
instances where a half-life changes, and these do not affect the dating
methods we have discussed. 1. Only one technical exception occurs
under terrestrial conditions, and this is not for an isotope used for dating.
According to theory, electron-capture is the most likely type of decay to
show changes with pressure or chemical combination, and this should be most
pronounced for very light elements. The artificially-produced isotope,
beryllium-7 has been shown to change by up to 1.5%, depending on its chemical
environment (Earth Planet. Sci. Lett. 171, 325-328, 1999; see also Earth
Planet. Sci. Lett. 195, 131-139, 2002). In another experiment, a
half-life change of a small fraction of a percent was detected when
beryllium-7 was subjected to 270,000 atmospheres of pressure, equivalent to
depths greater than 450 miles inside the Earth (Science 181,
1163-1164, 1973). All known rocks, with the possible exception of diamonds,
are from much shallower depths. In fact, beryllium-7 is not used for dating
rocks, as it has a half-life of only 54 days, and heavier atoms are even less
subject to these minute changes, so the dates of rocks made by
electron-capture decays would only be off by at most a few hundredths of a percent. 2. Physical conditions at the center of
stars or for cosmic rays differ very greatly from anything experienced in
rocks on or in the Earth. Yet, self-proclaimed "experts" often
confuse these conditions. Cosmic rays are very, very high-energy atomic
nuclei flying through space. The electron-capture decay mentioned above does
not take place in cosmic rays until they slow down. This is because the
fast-moving cosmic ray nuclei do not have electrons surrounding them, which
are necessary for this form of decay. Another case is material inside of
stars, which is in a plasma state where electrons are not bound to atoms. In
the extremely hot stellar environment, a completely different kind of decay
can occur. ' Bound-state beta decay' occurs when the nucleus emits an
electron into a bound electronic state close to the nucleus. This has been
observed for dysprosium-163 and rhenium-187 under very specialized conditions
simulating the interior of stars (Phys. Rev. Lett., 69, 2164-2167; Phys.
Rev. Lett., 77, 5190-5193, 1996). All normal matter, such as everything
on Earth, the Moon, meteorites, etc. has electrons in normal positions, so
these instances never apply to rocks, or anything colder than several hundred
thousand degrees. As an example of incorrect application
of these conditions to dating, one young-Earth proponent suggested that God
used plasma conditions when He created the Earth a few thousand years ago.
This writer suggested that the rapid decay rate of rhenium under extreme
plasma conditions might explain why rocks give very old ages instead of a
young-Earth age. This writer neglected a number of things, including: a)
plasmas only affect a few of the dating methods. More importantly, b) rocks
and hot gaseous plasmas are completely incompatible forms of matter! The
material would have to revert back from the plasma state before it could form
rocks. In such a scenario, as the rocks cooled and hardened, their ages would
be completely reset to zero as described in previous sections. If this
person's scenario were correct, instead of showing old ages, all the rocks
should show a uniform ~4,000 year age of creation. That is obviously not what
is observed. 3. The last case also involves very
fast-moving matter. It has been demonstrated by atomic clocks in very fast
spacecraft. These atomic clocks slow down very slightly (only a second or so
per year) as predicted by Einstein's theory of relativity. No rocks in our
solar system are going fast enough to make a noticeable change in their
dates. These cases are very specialized, and
all are well understood. None of these cases alter the dates of rocks either
on Earth or other planets in the solar system. The conclusion once again is
that half-lives are completely reliable in every context for the dating of rocks
on Earth and even on other planets. The Earth and all creation appears to be
very ancient. It would not be inconsistent with the
scientific evidence to conclude that God made everything relatively recently,
but with the appearance of great age, just as Genesis 1 and 2 tell of God
making Adam as a fully grown human (which implies the appearance of age).
This idea was captured by Phillip Henry Gosse in the book, "Omphalos:
An Attempt to Untie the Geological Knot", written just two years
before Darwin's "Origin of Species". The idea of a false
appearance of great age is a philosophical and theological matter that we
won't go into here. The main drawback--and it is a strong one--is that this
makes God appear to be a deceiver. However, some people have no problem with this.
Certainly whole civilizations have been incorrect (deceived?) in their
scientific and theological ideas in the past. Whatever the philosophical
conclusions, it is important to note that an apparent old Earth is
consistent with the great amount of scientific evidence. Rightly Handling the Word of
Truth As Christians it is of great importance
that we understand God's word correctly. Yet from the middle ages up until
the 1700s people insisted that the Bible taught that the Earth, not the Sun,
was the center of the solar system. It wasn't that people just thought it had
to be that way; they actually quoted scriptures: "The Earth is firmly
fixed; it shall not be moved" (Psalm 104:5), or "the sun stood still"
(Joshua 10:13; why should it say the sun stood still if it is the Earth's
rotation that causes day and night?), and many other passages. I am afraid
the debate over the age of the Earth has many similarities. But I am
optimistic. Today there are many Christians who accept the reliability of
geologic dating, but do not compromise the spiritual and historical inerrancy
of God's word. While a full discussion of Genesis 1 is not given here,
references are given below to a few books that deal with that issue.
APPENDIX: Common Misconceptions
Regarding Radiometric Dating Methods There are a number of misconceptions
that seem especially prevalent among Christians. Most of these topics are
covered in the above discussion, but they are reviewed briefly here for
clarity. 1. Radiometric dating is based on
index fossils whose dates were assigned long before radioactivity was
discovered. This is not at all true, though it is
implied by some young-Earth literature. Radiometric dating is based on the
half-lives of the radioactive isotopes. These half-lives have been measured
over the last 40-90 years. They are not calibrated by fossils. 2. No one has measured the decay
rates directly; we only know them from inference. Decay rates have been directly measured
over the last 40-100 years. In some cases a batch of the pure parent material
is weighed and then set aside for a long time and then the resulting daughter
material is weighed. In many cases it is easier to detect radioactive decays
by the energy burst that each decay gives off. For this a batch of the pure
parent material is carefully weighed and then put in front of a Geiger
counter or gamma-ray detector. These instruments count the number of decays
over a long time. 3. If the half-lives are billions of
years, it is impossible to determine them from measuring over just a few
years or decades. The example given in the section
titled, "The Radiometric Clocks" shows that an accurate
determination of the half-life is easily achieved by direct counting of
decays over a decade or shorter. This is because a) all decay curves have
exactly the same shape (Fig. 1), differing only in the half-life, and b)
trillions of decays can be counted in one year even using only a fraction of
a gram of material with a half-life of a billion years. Additionally, lavas
of historically known ages have been correctly dated even using methods with
long half-lives. 4. The decay rates are poorly known,
so the dates are inaccurate. Most of the decay rates used for dating
rocks are known to within two percent. Uncertainties are only slightly higher
for rhenium (5%), lutetium (3%), and beryllium (3%), discussed in connection with
Table 1. Such small uncertainties are no reason to dismiss radiometric
dating. Whether a rock is 100 million years or 102 million years old does not
make a great deal of difference. 5. A small error in the half-lives
leads to a very large error in the date. Since exponents are used in the dating
equations, it is possible for people to think this might be true, but it is
not. If a half-life is off by 2%, it will only lead to a 2% error in the
date. 6. Decay rates can be affected by
the physical surroundings. This is not true in the context of
dating rocks. Radioactive atoms used for dating have been subjected to
extremes of heat, cold, pressure, vacuum, acceleration, and strong chemical
reactions far beyond anything experienced by rocks, without any significant
change. The only exceptions, which are not relevant to dating rocks, are
discussed under the section, "Doubters Still Try", above. 7. A small change in the nuclear
forces probably accelerated nuclear clocks during the first day of creation a
few thousand years ago, causing the spuriously old radiometric dates of
rocks. Rocks are dated from the time of their
formation. For it to have any bearing on the radiometric dates of rocks, such
a change of nuclear forces must have occurred after the Earth (and the rocks)
were formed. To make the kind of difference suggested by young-Earth
proponents, the half-lives must be shortened from several billion years down
to several thousand years--a factor of at least a million. But to shorten
half-lives by factors of a million would cause large physical changes. As one
small example, recall that the Earth is heated substantially by
radioactive decay. If that decay is speeded up by a factor of a million or
so, the tremendous heat pulse would easily melt the whole Earth,
including the rocks in question! No radiometric ages would appear old if this
happened. 8. The decay rates might be slowing
down over time, leading to incorrect old dates. There are two ways we know this didn't
happen: a) we have checked them out with "time machines", and b) it
doesn't make sense mathematically. Both of these points are explained in the
section titled, "Can We Really Believe the Dating Systems?" 9. We should measure the
"full-life" (the time at which all of the parent is gone) rather
than the half-life (the time when half of it is gone). Unlike sand in an hourglass, which
drops at a constant rate independent of how much remains in the top half of
the glass, the number of radioactive decays is proportional to the amount of
parent remaining. Figure 1 shows how after 2 half-lives, 1/2 x 1/2 = 1/4 is
left, and so on. After 10 half-lives there is 2-10 = 0.098%
remaining. A half-life is more easy to define than some point at which almost
all of the parent is gone. Scientists sometimes instead use the term
"mean life", that is, the average life of a parent atom. The mean
life is always 1/ln(2) = 1.44 times the half-life. For most of us half-life
is easier to understand. 10. To date a rock one must know the
original amount of the parent element. But there is no way to measure how
much parent element was originally there. It is very easy to calculate the
original parent abundance, but that information is not needed to date the
rock. All of the dating schemes work from knowing the present abundances
of the parent and daughter isotopes. The original abundance N0, of
the parent is simply N0 = N ekt, where N is the present
abundance, t is time, and k is a constant related to the half life. 11. There is little or no way to tell
how much of the decay product, that is, the daughter isotope, was originally
in the rock, leading to anomalously old ages. A good part of this article is devoted
to explaining how one can tell how much of a given element or isotope was
originally present. Usually it involves using more than one sample from a
given rock. It is done by comparing the ratios of parent and daughter
isotopes relative to a stable isotope for samples with different relative
amounts of the parent isotope. For example, in the rubidium-strontium method
one compares rubidium-87/strontium-86 to strontium-87/strontium-86 for
different minerals. From this one can determine how much of the daughter
isotope would be present if there had been no parent isotope. This is the
same as the initial amount (it would not change if there were no parent
isotope to decay). Figures 4 and 5, and the accompanying explanation, tell
how this is done most of the time. While this is not absolutely 100%
foolproof, comparison of several dating methods will always show whether the
given date is reliable. 12. There are only a few different
dating methods. This article has listed and discussed a
number of different radiometric dating methods and has also briefly described
a number of non-radiometric dating methods. There are actually many more
methods out there. Well over forty different radiometric dating methods are
in use, and a number of non-radiogenic methods not even mentioned here. 13. "Radiation halos" in
rocks prove that the Earth was young. This refers to tiny halos of crystal
damage surrounding spots where radioactive elements are concentrated in
certain rocks. Halos thought to be from polonium, a short-lived element
produced from the decay of uranium, have been found in some rocks. A plausible
explanation for a halo from such a short-lived element is that these were not
produced by an initial concentration of the radioactive element. Rather, as
water seeped through cracks in the minerals, a chemical change caused
newly-formed polonium to drop out of solution at a certain place and almost
immediately decay there. A halo would build up over a long period of
time even though the center of the halo never contained more than a few atoms
of polonium at one time. "Hydrothermal" effects can act in ways that
at first seem strange, such as the well-known fact that gold--a chemically
un-reactive metal with very low solubilities--is concentrated along quartz
veins by the action of water over long periods of time. Other
researchers have found halos produced by an indirect radioactive decay effect
called hole diffusion, which is an electrical effect in a crystal. These
results suggest that the halos in question are not from short-lived isotopes
after all. At any rate, halos from uranium
inclusions are far more common. Because of uranium's long half-lives, these
halos take at least several hundred million years to form. Because of this,
most people agree that halos provide compelling evidence for a very old Earth. 14. A young-Earth research group
reported that they sent a rock erupted in 1980 from Mount Saint Helens
volcano to a dating lab and got back a potassium-argon age of several million
years. This shows we should not trust radiometric dating. There are indeed ways to
"trick" radiometric dating if a single dating method is improperly
used on a sample. Anyone can move the hands on a clock and get the wrong
time. Likewise, people actively looking for incorrect radiometric dates can
in fact get them. Geologists have known for over forty years that the
potassium-argon method cannot be used on rocks only twenty to thirty years
old. Publicizing this incorrect age as a completely new finding was
inappropriate. The reasons are discussed in the Potassium-Argon Dating
section above. Be assured that multiple dating methods used together on
igneous rocks are almost always correct unless the sample is too difficult to
date due to factors such as metamorphism or a large fraction of xenoliths. 15. Low abundances of helium in
zircon grains show that these minerals are much younger than radiometric
dating suggests. Zircon grains are important for
uranium-thorium-lead dating because they contain abundant uranium and thorium
parent isotopes. Helium is also produced from the decay of uranium and
thorium. However, as a gas of very small atomic size, helium tends to escape
rather easily. Researchers have studied the rates of diffusion of helium from
zircons, with the prediction from one study by a young-Earth
creationist suggesting that it should be quantitatively retained despite its atomic
size. The assumptions of the temperature conditions of the rock over time are
most likely unrealistic in this case. 16. The fact that radiogenic helium
and argon are still degassing from the Earth's interior prove that the Earth
must be young. The radioactive parent isotopes,
uranium and potassium, have very long half-lives, as shown in Table 1. These
parents still exist in abundance in the Earth's interior, and are still
producing helium and argon. There is also a time lag between the production of
the daughter products and their degassing. If the Earth were geologically
very young, very little helium and argon would have been produced. One can
compare the amount of argon in the atmosphere to what would be expected from
decay of potassium over 4.6 billion years, and in fact it is consistent. 17. The waters of Noah's flood could
have leached radioactive isotopes out of rocks, disturbing their ages. This is actually suggested on one
website! While water can affect the ability to date rock surfaces or other
weathered areas, there is generally no trouble dating interior portions of
most rocks from the bottom of lakes, rivers, and oceans. Additionally, if
ages were disturbed by leaching, the leaching would affect different isotopes
at vastly different rates. Ages determined by different methods would be in
violent disagreement. If the flood were global in scope, why then would we
have any rocks for which a number of different methods all agree with
each other? In fact, close agreement between methods for most samples is a
hallmark of radiometric dating. 18. We know the Earth is much
younger because of non-radiogenic indicators such as the sedimentation rate
of the oceans. There are a number of parameters which,
if extrapolated from the present without taking into account the changes in
the Earth over time, would seem to suggest a somewhat younger Earth.
These arguments can sound good on a very simple level, but do not hold water
when all the factors are considered. Some examples of these categories are
the decaying magnetic field (not mentioning the widespread evidence for
magnetic reversals), the saltiness of the oceans (not counting
sedimentation!), the sedimentation rate of the oceans (not counting
Earthquakes and crustal movement, that is, plate tectonics), the relative
paucity of meteorites on the Earth's surface (not counting weathering or
plate tectonics), the thickness of dust on the moon (without taking into
account brecciation over time), the Earth-Moon separation rate (not counting
changes in tides and internal forces), etc. While these arguments do not
stand up when the complete picture is considered, the case for a very old
creation of the Earth fits well in all areas considered. 19. Only atheists and liberals are
involved in radiometric dating. The fact is that there are a number of
Bible-believing Christians who are involved in radiometric dating, and who
can see its validity firsthand. A great number of other Christians are firmly
convinced that radiometric dating shows evidence that God created the Earth
billions, not thousands, of years ago. 20. Different dating techniques
usually give conflicting results. This is not true at all. The fact that
dating techniques most often agree with each other is why scientists tend to
trust them in the first place. Nearly every college and university library in
the country has periodicals such as Science, Nature, and
specific geology journals that give the results of dating studies. The public
is usually welcome to (and should!) browse in these libraries. So the results
are not hidden; people can go look at the results for themselves. Over a
thousand research papers are published a year on radiometric dating,
essentially all in agreement. Besides the scientific periodicals that carry
up-to-date research reports, specific suggestions are given below for further
reading, both for textbooks, non-classroom books, and web resources. Virtual Dating--a very helpful educational
course on half-lives and radioactive decay was put together by Gary Novak at
California State University in Los Angeles. This site has several interactive
web "workbooks" to help the reader understand various concepts
involved with radiometricdating. http://vcourseware5.calstatela.edu/VirtualDating Reasons to Believe--a Christian
ministry supporting the old-Earth viewpoint. Dr. Hugh Ross, the founder and head
of the ministry, holds a PhD in Astronomy. The ministry supports an accurate
interpretation of the Bible while also supportive of science as a tool to
study God's creation. American Scientific Affiliation
(ASA)--an umbrella organization of Christians in many different areas of the
sciences. Most of the members hold an old-Earth view, though membership is
open to anyone supporting their positional statement. This website has
numerous resources on theology and Bible-science issues. Affiliation of Christian Geologists
(ACG)--an organization of Geologists who are Christians. The ACG is
affiliated with the ASA (above). Lord I Believe--a site maintained by
Hill Roberts, a self-professed conservative Christian and a Physicist. There
is a wealth of information, including presentations on the interpretation of
Genesis chapters 1-3, a resource list of apologetics ministries, etc. A review of Phillip Henry Gosse's Omphalos:
An Attempt to Untie the Geological Knot, in which fiat creation with the
appearance of age is suggested. Reviewed by Rev. John W. Burgeson. http://www.burgy.50megs.com/omphalos.htm Origins--this site is devoted mainly to
evidences for intelligent design in nature. Talk Origins--an archive dedicated to
creation-evolution issues. Originally created by Chris Stassen, this site is
supported by the National Center For Science Education. A Radiometric Dating Resource List--a
very comprehensive resource list for radiometric dating, maintained by Tim
Thompson of the NASA Jet Propulsion Laboratory. It includes separate resource
sections on the reliability of radiometric dating, introductory articles,
advanced articles, radiocarbon dating, etc. www.geocities.com/CapeCanaveral/8851/radiometric.html C-14 Dating--The radiocarbon
laboratories at Oxford (England) and Waikato (New Zealand) Universities
jointly operate this website which gives very comprehensive information on
radiocarbon dating. Portions of it were written specifically for use by K-12
students, so it is easy to understand. The site contains explanations on
measurements, applications, calibration, publications, and other areas. Cornell University Geology 656 Lecture
Notes--A large number of pdf files of geology lecture notes are available on
the web. These are university-level lecture notes describing radiometric
dating and related topics. http://www.geo.cornell.edu/geology/classes/Geo656/656notes98.html http://www.geo.cornell.edu/geology/classes/Geo656/656notes00.html Further
Reading: Books Radiometric
dating textbooks: The following books
are popular college-level Geology texts that deal in depth with various
dating techniques. Geologic Time is very easy to read and has been
around for quite some time. The text by Dalrymple is meant to be relatively
easy to read, but is also very comprehensive. The Faure and Dickin texts are
regular textbooks for Geology, including more mathematics and more details. Dickin, Alan P. (1995) Radiogenic
Isotope Geology. Cambridge University Press, 490 pp. Dalrymple, G. Brent (1991) The Age
of the Earth. Stanford University Press, 474 pp. Faure, Gunter (1991) Principles and
Applications of Inorganic Geochemistry: AComprehensive Textbook for Geology
Students. MacMillan Pub. Co., New York, 626 pp. Faure, Gunter (1986) Principles of
Isotope Geology, 2nd edition. Wiley, New York, 464 pp. Eicher, Don L. (1976) Geologic Time,
2nd edition. Prentice-Hall, Englewood Cliffs, NJ, 150 pp. Jespersen, James, and Jane
Fitz-Randolph (1996) Mummies, Dinosaurs, Moon Rocks: How We Know How Old
Things Are. Atheneum Books, New York, 92 pp. This
is a book designed for easy reading on the general subject of dating. This
short book covers topics from archeology to tree ring dating to radiocarbon
dating of the dead sea scrolls, to dating of meteorites and moon rocks. The
book is out of print, but slightly used copies can be obtained from online
dealers like Amazon. Wagner, G?nther A. (1998) Age
Determination of Young Rocks and Artifacts. Springer-Verlag,
New York, 466 pp. [Translated from the original Altersbestimmung von
jungen Gesteinen und Artefakten, Ferdinand Enke Verlag, Stuttgart, 1995] This
book is a quite comprehensive reference on all methods for determining dates
less than about a million years old. It includes a large amount of
information on archeological dating, and describes more methods than are
discussed here, including TL, ESR, racemization, fluorine/uranium/nitrogen
uptake, cosmic-ray exposure-age, fission track, radiocarbon, and others. Strahler, Arthur N. (1987) Science
and Earth History--The Evolution/Creation Controversy. Prometheus Books,
Buffalo, 552 pp. This
book is a very thorough and comprehensive refutation of young-Earth ideas,
written by a non-Christian. The only negative aspect is that at one point
Strahler throws in a bit of his own theology--his arguments against the need
for a God. This book is long and in small print; it covers a wealth of
information. For ice core studies, the Journal of
Geophysical Research, volume 102, (1997) starting with page 26,315, has 47
papers on two deep ice cores drilled in central Greenland. Books
on scripture, theology, and science: Snoke, David (1998) A Biblical Case
for an Old Earth. Interdisciplinary Biblical Research Institute (IBRI),
Hatfield, PA, 76 pp. Dr.
Snoke, an elder in the Presbyterian Church (PCA) and a Physics professor,
presents a strong case for a geologically old Earth. He addresses typical
objections brought up by young-Earth adherents, including the death of
animals before Adam and Eve's sin, entropy (or decay) before the fall, the six
days of creation, and the flood. Sailhamer, John (1996) Genesis
Unbound. Multnomah Books, Sisters, OR, 257 pp. This
is a very readable theological book about Genesis. Dr. Sailhamer has served
on the translation committees for two versions of the book of Genesis. He has
taught at Bethel Seminary, Philadelphia College of the Bible, Trinitiy
Evangelical Divinity School, Northwestern College, and Western Seminary. Ross, Hugh (1994) Creation and Time:
A Biblical and Scientific Perspective on the Creation-Date Controversy. NavPress,
Colorado Springs, CO. Hugh
Ross has a PhD in Astronomy. In this book Dr. Ross defends modern science and
an old age for the universe, and refutes common young-Earth arguments. He
firmly believes in the inerrancy of the Bible. Stoner, Don (1992) A New Look at an
Old Earth. Schroeder, Paramount, CA, 191 pp. A
persuasive book written for the Christian layman. Stoner uses arguments both
from the theological and the scientific side. He talks somewhat
philosophically about whether God deceives us with the Genesis account if the
Earth is really old. Stoner also tries to discuss the meaning of the Genesis
1 text. Van Till Howard J., Young Davis A., and
Menninga Clarence (1988) Science Held Hostage. InterVarsity, Downers
Grove, IL, 189 pp. This
book talks about the misuse of science by both hard-line atheists and by
young-Earth creationists. A good deal of the book is devoted to refuting
young-Earth arguments, including a substantial section on the Grand Canyon
geology. Its authors are well-known Christians in Geology and Physics. Wiester, John (1983) The Genesis
Connection. Interdisciplinary Biblical Research Institute, Hatfield, PA,
254 pp. John
Wiester has taught Geology at Westmont and Biola University, and is active in
the American Scientific Affiliation, an organization of scientists who are
Christians. This book discusses many scientific discoveries relating to the
age of the Earth and how these fit into the context of Genesis 1. Young, Davis A. (1982) Christianity
and the Age of the Earth. Zondervan, Grand Rapids, MI (now available
through Artisan Sales, Thousand Oaks, CA). Davis
Young has a PhD in Geology and teaches at Calvin College. He argues for an
old Earth and refutes many of the common young-Earth claims (including their
objections to radiometric dating). Acknowledgements: A number of members of the American Scientific
Affiliation and other Christians involved in the sciences reviewed this paper
and/or made contributions. The following people are sincerely thanked for
their contributions to the first edition: Drs. Jeffery Greenberg and Stephen
Moshier (Wheaton College), John Wiester (Westmont College), Dr. Davis Young
(Calvin College), Dr. Elaine Kennedy (Loma Linda University), Steven
Schimmrich (U. of Illinois), Dr. Kenneth VanDellen (Macomb Community
College), Dr. Guillermo Gonzalez (U. Texas, Austin), Ronald Kneusel, and
James Gruetzner (U. New Mexico). The second edition, likewise, was
significantly improved through reviews by Carol Ann Hill, Hill Roberts,
Professor Jeffrey Greenberg (Wheaton College), Ken Wohlgemuth, and Dr.
Kenneth Van Dellen. I thank my wife Gwen, and children, Carson and Isaac, for
supporting me in this work, and I thank God for giving us the intelligence to
understand little bits and pieces of His amazing creation. More about the
author: Dr. Wiens
received a bachelor's degree in Physics from Wheaton College and a PhD from
the University of Minnesota, doing research on meteorites and moon rocks. He spent
two years at Scripps Institution of Oceanography (La Jolla, CA) where he
studied isotopes of helium, neon, argon, and nitrogen in terrestrial rocks.
He worked seven years in the Geological and Planetary Sciences Division at
Caltech, where he continued the study of meteorites and worked for NASA on
the feasibility of a space mission to return solar wind samples to Earth for
study. Dr. Wiens wrote the first edition of this paper while in Pasadena. In
1997 he joined the Space and Atmospheric Sciences group at Los Alamos
National Laboratory, where he has been in charge of building and flying the
payload for the solar-wind mission, as well as developing new instruments for
other space missions. He has published over twenty scientific research papers
and has also published articles in Christian magazines. Dr. Wiens became a
Christian at a young age, and has been a member of Mennonite Brethren,
General Conference Baptist, and Conservative Congregational, and Vineyard
denominations. He does not see a conflict between science in its ideal form
(the study of God's handiwork) and the Bible, or between miracles on the one
hand, and an old Earth on the other. Alpha decay Radioactive decay in which the atom's nucleus emits an alpha
particle. An alpha particle consists of two neutrons and two protons--the
same as a helium atom nucleus. In alpha decay, the daughter is four atomic
mass units lighter than the parent. Alpha decay is most common in heavy
elements. Atom
The smallest unit that materials can be divided into. An atom is about ten
billionths of an inch in diameter and consists of a nucleus of nucleons
(protons and neutrons) surrounded by electrons. Beta decay Radioactive decay in which the atom's nucleus emits or
captures an electron or positron. The daughter ends up with the same mass as
the parent, but ends up with one more neutron and one less proton, or vice
versa. Because of the different number of protons, the daughter is a
different element with different chemical properties than the parent. Bound-state beta decay A special kind of beta decay in which an electron is
given off by the nucleus, and the electron ends up in an inner orbital, or
electron shell. This kind of decay only occurs if the nucleus is stripped of
the electrons that would normally be in the inner electron shells. As such,
this decay only occurs in the center of stars, and was only confirmed
experimentally in the 1990s. Calibration The cross-checking of one measurement with another,
usually more certain measurement. Essentially every method of measurement,
whether a thermometer, a ruler, or a more complicated instrument, relies on
calibration for accuracy. Carbonate A term used rather loosely in this context to describe
deposits containing the carbonate anion. Carbonates play an important role in
many caves, where cave formations are the result of dissolution and
re-precipitation of material interacting with carbonic acid. Carbonates in
recent cave deposits are useful because of their high carbon content, which
can be used to calibrate radiocarbon with uranium-series ages. Closed system A system (rock, planet, etc.) which has no influence or
exchange with the outside world. In reality there is always some exchange or
influence, but if this amount is completely insignificant for the process
under consideration (e.g., for dating, if the loss or gain of atoms is
insignificant) for practical purposes the system can be considered closed. Cosmic ray A very high-energy particle which flies through space.
Cosmic Rays are stopped by the Earth's atmosphere, but in the process, they
constantly produce carbon-14, beryllium-10, chlorine-36, and a few other
radioactive isotopes in small quantities. Cosmic-ray exposure dating Dating of surfaces exposed to cosmic rays by measuring the
neon-21, helium-3, or other cosmogenic isotopes produced in rocks or
meteorites exposed to cosmic rays. Cosmogenic Produced by bombardment of cosmic rays. Carbon-14 is said
to be cosmogenic because it is produced by cosmic rays hitting the Earth's
atmosphere. Daughter The element or isotope which is produced by radioactive
decay. Decay
The change from one element or isotope to another. Only certain isotopes
decay. The rest are said to be stable. Dendrochronology The counting of yearly growth rings on trees. A continuous
record of growth rings has been used to calibrate radiocarbon ages back as
far as 10,000 years ago. "Floating" dendrochronologies
(non-continuous records) go back farther in time. Deposit Mineral
or sandy matter settled out of water or accumulated in a vein. Deuterium 'Heavy hydrogen'; the heavy isotope of hydrogen which
contains one proton and one neutron, as compared with only a single proton in
normal hydrogen. Water consists of molecules mostly containing normal hydrogen,
but with a few molecules containing deuterium. Electron-capture decay The only type of radioactive decay that requires the
presence of something--an electron--outside of the atom's nucleus. Electron
capture decay of light atoms--those having the fewest electrons--can be very
slightly affected by extremely high pressures or certain chemical bonds, so
as to change their half-lives by a fraction of a percent. But no change in
the half-lives of elements used for radiometric dating has ever been verified. Element A
substance that has a certain number of protons in the nucleus. Each element
has unique properties. Elements may be further broken down into isotopes,
which have nearly all of the same properties except for their mass and their
radioactive decay characteristics. Extinct Once
in existence, but no longer existing in nature. Radioactive Subject to change from one element to another. During the
change, or decay, energy is released either in the form of light or energetic
particles. Radiocarbon Carbon-14, which is used to date dead plant and animal
matter. Radiocarbon is generally not used for dating rocks. Radiometric dating Determination of a time interval (e.g. the time since
formation of a rock) by means of the radioactive decay of its material.
Radiometric dating is one subset of the many dating methods used in geology. Stalactite A cylindrical or conical deposit of minerals, generally
calcite or aragonite (forms of calcium carbonate), hanging from the roof of a
cavern, and generally formed by precipitation (or crystallization) of
carbonates from water dripping from the roof. Stalagmite Columns or ridges of carbonate rising from a limestone
cave floor, and formed by water charged with carbonate dripping from the stalactites
above. Thermoluminescence (TL) dating A method of dating minerals and pottery. Rather than relying on a
half-life, this method relies instead on the total amount of radiation
experienced by the mineral since the time it was formed. This radiation causes disorder in
the crystals, resulting in electrons dwelling in higher orbits than they
originally did. When the
sample is heated in the laboratory in the presence of a sensitive light
detector, these electrons return to their original orbits, emitting light and
allowing an age to be determined by comparison of the amount of light to the
radioactivity rate experienced by the mineral. Variations on this method include optically-stimulated
luminescence (OSL) and infrared-stimulated luminescence (IRSL) dating. Three-isotope plot In dating, this is a plot in which one axis represents
the parent isotope and the other axis represents the daughter isotope. Both parent and daughter isotopes
are ratioed to a daughter-element isotope that is not produced by radioactive
decay. So the vertical axis
gives the daughter/stable ratio while the horizontal axis gives the
parent/stable isotope ratio. This
type of plot gives the age independent of the original amounts of the
isotopes. Tree ring A ring visible in the sawed or cored section of a tree
which indicates how much it grew in a year. The age of a tree can be determined by counting the growth
rings. Two-component mixing The mixing of two different source materials to produce a
rock. On rare occasions this
can result in an incorrect age for certain methods that use three-isotope
plots. Two-component mixing
can be recognized if more than one dating method is used, or if surrounding
rocks are dated. Uranium-series decay chain The decay of the long-lived uranium-238 and -235 and
thorium-232 which produce shorter-lived radioactive daughters, each of which
decay to lighter radioactive elements until they eventually end up as various
stable isotopes of lead. Varve A
sedimentary layer showing distinct texture or color for different seasons
within a single year. Varve
layers can be counted like tree rings. Xenolith
Literally, a foreign chunk of rock within a rock. Some rocks contain pieces of older rocks within them. These pieces were ripped off of
the magma chamber in which the main rock formed and were incorporated into
the rock without melting. Xenoliths
do not occur in most rocks, and they are usually recognizable by eye where
they do occur. If
unrecognized, they can result in an incorrect date for a rock (the date may be
of the older xenolith). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||