|
A Plant Defense Dimorphism in
Western Jimsonweed, Datura wrightii |
||||
|
|
||||
|
I had
considered several natural systems to study the ecology and evolution of
plant-insect and tritrophic interactions.
One that interested me focused upon the native plant species, Datura wrightii, or Western
Jimsonweed. Local populations of Datura wrightii consist of some plants
with leaves covered with long glandular trichomes, or plant hairs, that
secrete sticky substances (Figs. 1 and 3) whereas other plants in the same
population produce leaves having a high density of relatively short,
nonglandular trichomes (Figs. 2 and 4).
This dimorphism was associated with differences in susceptibility to
different species of insect herbivores, and we began detailed studies on this
system in the mid 1990’s. I am
indebted to former postdocs at the time, Drs. Nicole
van Dam and Elizabeth Elle
for their careful and enthusiastic participation in this project. |
||||
|
Figure 1. Lower
surface of a leaf of Datura wrightii
showing long, glandular trichomes on the leaf surface and veins. Leaves feel sticky when touched. |
Figure 2. Lower
surface of a leaf of Datura wrightii
showing short, nonglandular trichomes on the leaf surface and veins. Leaves feel velvety when touched. |
|||
|
Figure 3.
Electron micrograph of the Type IV glandular trichomes of a sticky D. wrightii leaf.
Also shown is a single Type VI four-lobed trichome. From van Dam et al. 1999. |
Figure 4. Electron
micrograph of the nonglandular trichomes of a D. wrightii velvety leaf.
Also shown is a Type VI four-lobed trichome for scale. From van Dam et al. 1999. |
|||
|
Genetics and Ontogeny of the Trichome Dimorphism |
||||
|
For
convenience, we refer to the two phenotypes largely by how the leaves feel when
touched; the leaves with nonglandular trichomes feel “velvety” whereas the
leaves with glandular phenotype feel “sticky.” The dimorphism has a simple genetic basis
with the “sticky” condition being dominant over the “velvety” condition, but
with an interesting developmental component.
As seedlings, all plants produce leaves covered by glandular
trichomes, but homozygous recessive plants begin producing leaves with an
increasing proportion of nonglandular trichomes until, after about eight
weeks, those plants produce only leaves with nonglandular trichomes (Fig. 5) [1]. |
||||
|
Figure 5. Ontogenetic
trajectory of leaf trichome morphology as a function of plant age for
homozygous recessive (velvety) plants.
From van Dam et al. 1999. |
||||
|
Characterization of the Sticky Exudate.
Like many other solanaceous plants with Type I and IV glandular trichomes, the glands at the tip of the trichomes secrete esters of sugars and simple aliphatic acids. In Datura, these esters, or acyl sugars, are comprised of glucose and C6- C9 aliphatic acids [2]. |
||||
|
Role of Glandular Trichomes and their Acyl Sugars in Resistance to Herbivores. The number of species within the herbivore community of Datura in southern California is relatively small (Figs. 6 – 12), but the trichomes and their acyl sugars have varying effects on the different species. Sticky plants are completely resistant to whiteflies (Fig. 6) [3]. The acyl sugars retard growth of the tobacco hornworm, Manduca sexta, when incorporated into an artificial diet [2]. Feeding by adult flea beetles (Fig. 8) and a seed weevil (Fig. 9) are moderately reduced when feeding on plants with acyl sugars, but feeding by larvae of a third beetle, the most abundant herbivore, Lema daturaphila (Fig. 10) is unaffected by trichome morphology or acyl sugar concentration [4]. Finally, Tupiocoris notatus, the most common insect found on sticky plants (Figs. 11, 12), uses the acyl sugars as both a feeding and oviposition stimulant [3]. The
varying responses of different species of herbivore to glandular trichomes
and their acyl sugars suggested that the value of glandular trichomes as a
resistance character could vary with the structure of the herbivore community
and the proportions of herbivore species differentially adapted to glandular
trichomes in different plant populations. |
||||
|
Figure 6.
Whiteflies (Trialeurodes
sp.) "stuck" to a sticky D. wrightii leaf. |
Figure 7. Although
charismatic and highly damaging, Manduca sexta are relatively infrequent herbivores of Datura in southern California. |
Figure 8. Adult
flea beetles (Epitrix spp.) and
their characteristic "pit feeding" damage to a velvety D. wrightii leaf. |
Figure 9. Adult Trichobaris compacta weevil and the characteristic, distributed "pit feeding" damage to a velvety D. wrightii leaf. |
|
|
Figure 10. Adult Lema daturaphila, the most abundant
herbivore of D. wrightii in
southern California. |
Figure 11. Adult Tupiocoris notatus shown feeding on a sticky D. wrightii leaf. |
Figure 12. Nymphs of Tupiocoris notatus shown on the lower surface of a sticky D. wrightii leaf. Leaf stippling is characteristic consequence of feeding by this sucking insect. |
||
|
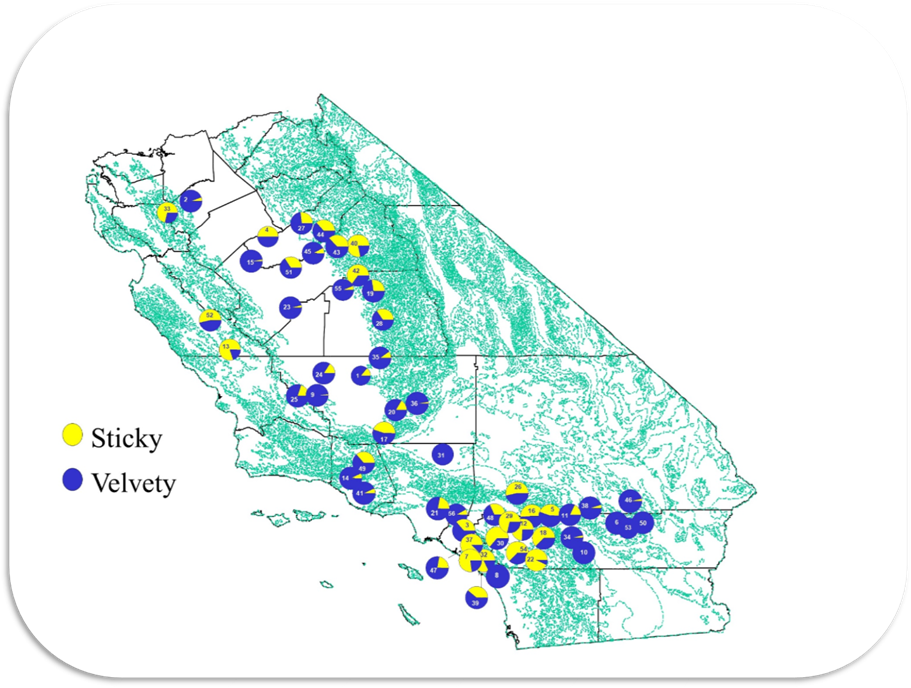
Geographic Variation in the Frequencies of Plants with Glandular and Nonglandular Trichomes. Although D. wrightii can be found in most
counties in central and southern California, it has a patchy distribution and
occurs in relatively small, widely separated populations. Censuses of several populations during the
late 1990’s showed that the frequency of the two trichome phenotypes varied
substantially, (Fig. 13) ranging from a high of 93% sticky plants near Lake
Elsinore to 100% velvety plants in the Mojave desert* [1, 5]. |
||||
|
Figure 13.
Frequencies of sticky and velvety D. wrightii plans in central and southern California. From Hare and Elle 2001. *Colleagues report only the velvety
phenotype occurring in Arizona and Utah. |
||||
|
Potential Physiological Role of Trichomes in Gas Exchange In some plant species in arid environments, trichomes can help protect plants from excessive heat and water loss. We investigated this directly but found minimal differences in temperatures of leaves of sticky and velvety plants, and no significant differences in gas exchange [6]. Thus, for D. wrightii the differences in trichome morphology may be more likely associated with defense against herbivores than with physiological adaptations to arid environments. With this background information, our next objective was to examine the costs and benefits of glandular trichome production in natural habitats where all plants were exposed to herbivores and under experimental field conditions in which plants were either subjected to natural herbivory or protected from herbivores. |
||||
|
1
van Dam, N.M., et al. (1999)
Inheritance and distribution of trichome phenotypes in Datura wrightii. J. Hered. 90, 220-227. DOI: 10.1093/jhered/90.1.220 2 van Dam, N.M. and Hare, J.D. (1998) Biological activity
of Datura wrightii glandular trichome exudate against Manduca sexta larvae. J.
Chem. Ecol. 24, 1529-1549. DOI: 10.1023/A:1020963817685 3 van Dam, N.M. and Hare, J.D. (1998) Differences in
distribution and performance of two sap-sucking herbivores on glandular and
non-glandular Datura wrightii. Ecol. Entomol. 23, 22-32. DOI: 10.1046/j.1365-2311.1998.001DOI:
10.x 4 Hare, J.D. (2005) Biological activity of acyl glucose
esters from Datura wrightii glandular trichomes against
three native insect herbivores. J.
Chem. Ecol. 31, 1475-1491. DOI: 10.1007/s10886-005-5792-1 5 Hare, J.D. and Elle, E. (2001) Geographic variation in
the frequencies of trichome phenotypes of Datura
wrightii and correlation with
annual water deficit. Madrono 48,
33-37. JSTOR: 41425387. 6 Smith, J.L., II and Hare, J.D. (2004) Spectral
properties, gas exchange, and water potential of leaves of glandular and
non-glandular trichome types in Datura
wrightii (Solanaceae). Funct. Plant
Biol. 31, 267-273.
DOI: 10.1071/fp03178 |
||||