File: <filariasis.htm> <General Index> Site Description Glossary <Navigate to Home>
|
FILARIASIS (Contact) Please CLICK on
underlined links for details: There are
many vectors of Filariasis
among the mosquito genera Anopheles,
Mansonia, Coquillettdia and Culex. Also some Culicoides spp.
are vectors of filarial parasites to humans (See Matheson 1950 for a long
list). Mansonella
perstans of West and Central Africa is
vectored by Culicoides
milnei and C.
austeni and probably also C.
grahamsii. Breeding is in
rotting stumps of bananas. Mansonella perstans also occurs in
Central and South America where the vectors are other species of Culicoides. Mansonella
ozzardi occurs from Mexico to Panama, the Caribbean and South
America. Vectors are Culicoides furens, C. phlebotomus and other Culicoides spp. as well as some
Simuliidae. Mansonella
streptocerca is a species found in Central and
West Africa. The principal vector is Culicoides grahamii, but C. milnei and C. austeni are also suspected (Service
2008). Wuchereria
bancrofti incites most cases of filarial
infection in humans. It occurs over
much of the tropics and subtropics of South American, central and southern
Africa, and Asia and the South Pacific.
Matheson (1950) reported that the adult worms live together,
frequently coiled up in various parts of the lymphatic system. The females discharge their embryos in the
lymph channels from which they gain access to the blood stream. The embryos are called
"microfilariae." There is a
periodicity in the appearance of the microfilariae, the maximum nocturnal
abundance occurring between 10 PM and 2 AM, while in daytime they concentrate
in the pulmonary vessels, heart capillaries and kidneys. In the Pacific area there is also a
nonperiodic strain, the microfilariae being present in the blood stram of
infected humans at all times during the day as well as the night. When a mosquito
obtains blood infected with microfilariae, the embryos escape from their
sheaths and bore through the intestinal wall. After 24 hours most have migrated to the thoracic muscles where
each worm undergoes further development without an increase in numbers. Then from 11-20 days the larval
development is complete and the parasites migrate forward to the mosquito's
proboscis. Later they end up in the hemocele
of the labium from which they are set to pass to a new host. When the mosquito takes blood the worms
escape from the labium and bore directly through the human's skin. Afterwards the larvae reach the lymphatics
where they become sexually mature and new generations of microfilariae enter
the blood stream (Matheson 1950). The
mosquito is important in the development and transfer of the roundworm. Temperature and humidity determine whether
a mosquito becomes infected, as was demonstrated by Basu & Rao (1939). They found although almost 100 percent
infection will occur at 80-deg. F., and Relative humidity of over 90 percent,
but at temperatures under 60-deg. F. and low humidity infection rarely
occurs. In cases where infection does
occur at the lower temperature the developmental period in the mosquito was
much prolonged. There are many different species of
mosquito that can act as intermediate hosts in the developmental cycle of Wuchereria. In 1950 Matheson listed the following species, but noted that
more species are certainly involved.
The acceleration of world trade in the 21st Century can also be
expected to distribute species to different world sites.
Infection with filarial worms in humans does not always
cause an apparent disease expression.
But there may be marked changes in the lymphatic system that cause
serious health problems among which are lymphangitis,
adenitis and elephantiasis. As of 2016 there are no known effective treatments other than
mosquito control for Filariasis. Avoidance of geographic areas where the
disease is prevalent, such as the Marquesas
Islands of the southern Pacific, is a precautionary measure. The principal
species of Flavivirus involved
in Filariasis were listed by
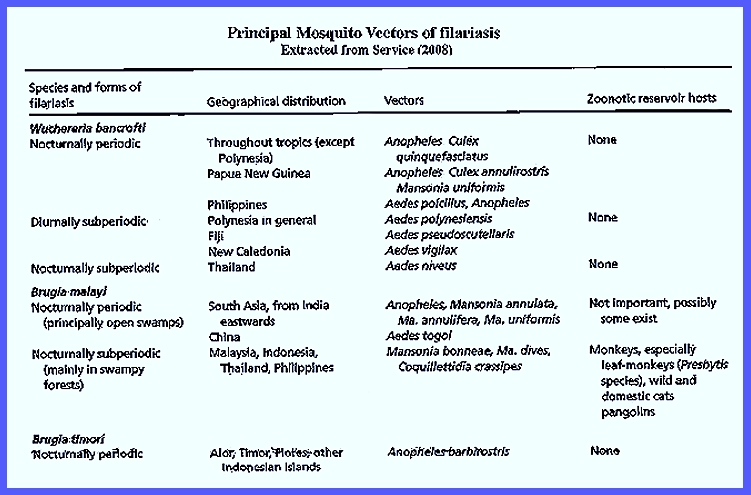
Service (2008) as shown in the following table: CLICK To Enlarge Service
(2008) reported that Wuchereria bancrofti occurs
in tropical areas of the world as the most common filarial human
infection. Bancroftian Filariasis is is found mostly in urban areas
with no animal reservoir hosts. In the nocturnal periodic form Culex
quinquefasciatus breeds in polluted water in Asia, South
America and Africa vectors the nocturnal periodic form. Adult mosquitoes bite during nighttime and
subsequently rest in dwellings. Although
Cx. quinquefasciatus
is an efficient vector in Africa, Anopheles
gambiae and An.
funestus are the main vectors in the western portion of that
continent. Various other mosquito
species transmit the virus in Asia and New Guinea (e.g., Anopheles spp, Mansonia uniformis
and Culex annulirostris). Aedes poicilius
is the main vector in the Philippines, which bites at dusk. Their larvae develop in the leaf axils of
bananas and coco yams. Only the diurnal subperiodic form exists in
Polynesia where the main vector is Aedes
polynesiensis that bites during daytime. Their larvae develop in natural
containers, coconut shells, crab holes and various human made
receptacles. Aedes pseudoscutellaris in Fiji oviposits
in tree holes and bamboo stumps with larval development being in crab
holes. In New Caledonia Aedes vigilax is a daytime biter, and
their larvae develop in pools of standing water. In Thailand
the nocturnal subperiodic form
involves the Aedes niveus complex,
which breed primarily in bamboo. Occurring
through most of Asia the nocturnal
periodic form is primarily a disease in rural areas without any known
animal reservoirs. The vectors, which
bite both during the day or night, include Anopheles
and Mansonia mosquitoes that
bite mainly during the night (e.g., Mansonia
annulifera of India and Mansonia uniformis
elsewhere breeding in permanent water).
In Malaysia, Indonesia, Thailand and the Philippines this form is
vectored by Mansonia (e.g., Mansonia dives, Mansonia bonneae and Mansonia annulifera). Coquillettidia
crassipes is active in the Philippines also. Reservoir hosts are wild simians, and
humans become infected when encountering them in forests (Service 2008). Control Service
(2008) listed a number of ways to control the disease, all involving
avoidance of the vector mosquitoes.
He emphasized that it is more difficult to protect against the
culicine mosquitoes than the anophelines because many species are outdoor
biters during the day. Insecticidal
control is not very effective against culicines. Therefore, control of larvae is the most effective approach,
which involves the application of insecticides. = = = = = = = = = = = =
= = = = = = = = Key References: <medvet.ref.htm> <Hexapoda> Basu, B. C. & R. S. Rao. 1939. Studies on Filariasis. Indian J. Med. Res. 27: 233-49. Dobson, M. 2001.
Lymphatic Filariasis:
The Quest to Eliminate a 4,000-Year-Old Disease. Hollis Pub. Co., Hollis, New Hampshire. Matheson, R. 1950. Medical Entomology. Comstock Publ. Co, Inc. 610 p. Ottesen, E. A. 2003.
Lymphatic Filariasis:
tratment, control and elimination.
Adv. in Parasitol. 61: 1-47. Reeves, W. C.
1990. Epidemiology &
Control of Mosquitopborne Arboviruses in California, 1943-1987. California Mosquito & Vector Control
Assoc., Sacramento, CA. Service, M. 2008.
Medical Entomology For Students.
Cambridge Univ. Press. 289 p Legner, E.
F.
1995. Biological
control of Diptera of medical and veterinary importance. J. Vector Ecology 20(1): 59_120. Legner, E. F. 2000.
Biological control of aquatic Diptera. p. 847_870.
Contributions to a Manual of Palaearctic Diptera, Vol. 1, Sci. Herald, Budapest. 978 p. Muller, R. 2002.
Worms and Human Diseases. 2nd ed., CABI, Wallingford, England. Sasa, M.
1976. Human Filariasis: a Global Survey of
Epidemiology & Control. Univ. of
Tokya Press. White, G. B. & M. B. NathAn.
2002. The elimination of
lymphatic Filariasis:
public-health challenges and the role of vector control. Ann. Trop. Med. Parasit. 96: 1-164. World Health
Organization. 2005. Global programme to eliminate lymphatic Filariasis. Weekly Epidemiol. Rec. 80:
202-12. Zagaria, N. & L.
Savioli. 2002. Elimination of lymphatic filariasis: a
public health challenge. Ann. Trop
Med. & Parasit. 96(suppl. 2):
3-13. |