File:
<medvet.htm> <Home>
[ To search for Subject Matter, depress Ctrl/F
]: [ Please refer also to Related Research ]
|
BIOLOGICAL
CONTROL OF PESTS OF MEDICAL AND
VETERINARY IMPORTANCE Introduction
Mosquitoes Synanthropic Diptera Hymenoptera Snails
References
The manipulative use of natural enemies for the control of
medical and veterinary invertebrate pests has been restricted largely to
various species of Diptera. Some work has been conducted on ants,
cockroaches, wasps, ticks, and snails, but work on these animals has been
limited. Here are reviewed the biological control agents that can be
manipulated, agents that have been used successfully, agents that are being
researched and agents that show at least some promise for successful
application. Aquatic vegetation supporting mosquito development
Left Figure = Potamogeton weeds clogging an
irrigation canal siphon at Blythe, California.; Middle Figure = Hydrilla weeds dredged from
the All American Canal, Imperial Valley, California.; Right Figure = Potamogeton removed from the
lower half of a canal in the Coachella Valley, California, causes a cascade
of water, 4-ft. high at the unremoved portion upstream
Bay et al (1976) indicate that medically important pests differ from
agricultural pests in fundamental ways: First, pests that affect humans are
usually in the adult stage while those that attack crops are usually in the
immature stage. This is of some advantage for control of medically important
pests since it allows the control action to be taken against the immatures,
thus eliminating the adult before it can cause problems. A second difference,
however, is not favorable as it relates to setting tolerance levels. Whereas,
an allowable number of pests (tolerance level) can be established for the
biological control of a crop pest, it is far more difficult to establish for
pests attacking humans. For example, an individual mosquito can be of great
annoyance and can precipitate a reaction for control. In addition, low
population levels of a vector may still transmit a disease and, therefore,
cannot be tolerated (Service 1983). However, setting tolerance levels for
veterinary pests would be more in line with those for agricultural pests. A
third difference, usually a distinct disadvantage for biological control, is
that the habitat utilized by medically important pests is frequently
temporary as opposed to that of an agricultural crop which is more permanent.
In the agricultural situation, natural enemies can coexist with pests and
thus may regulate the pest populations. Additionally, in many situations the
habitat exploited by the medically important pests is only an undesirable
extension of human activity. An example would be the cultivation of rice,
where the production of pests such as mosquitoes is usually of little concern
to the grower.
Interest in biological control of medical pests and vectors had its modest
beginning in the late 1800\'s (Lamborn 1890). At that time the possible use
of dragonflies as natural enemies for the control of mosquitoes was clearly
recognized. However, as is true even today, the enormous difficulties
associated with the colonization and management of these insects quickly
extinguished any idea for the practical use of these predators for mosquito
control. In the early 1900\'s the mosquitofish, Gambusia affinis
(Baird & Girard), became stressed for biological control. This small
fish, being much easier to deal with than dragonflies, was quickly utilized
and transported throughout the world during the early decades of this century
in attempts to control mosquitoes (Legner & Sjogren 1984).
The mosquitofish, G. affinis, and a few other
natural enemies were employed with some vigor until the 1940\'s. All of these
control measures were curtailed sharply with the introduction of synthetic
organic insecticides after World War II. The convenience and quick killing
power of these chemicals was so dramatic for mosquitoes, flies and lice, that
other control tactics were quickly reduced to a minor role. Interest in
biological control, arose again when the succession of chemicals developed
during the 1940s and 1950s began to fail, due to the development of genetic
resistance in vector and pest populations. The biological control of
medically important pests and vectors has made slow progress since its
revival, behind that which has occurred in agricultural systems (Service
1983). This disparity is due to the problems of establishing pest tolerance
levels, and the temporary unstable habitats exploited by medically important
pests (Legner & Sjogren 1984).
While progress in the development of biological control agents has been
substantial and work in progress appears promising, an overall evaluation at
this point is that biological control will rarely be a panacea for medically
important pests. However, with continued effort it can be a major component
in the overall strategy for the control of some of these important pests
(Legner & Sjogren 1984).
The literature reviewed in this section according to major taxonomic groups
where some success has been achieved or where work is currently being
conducted are the mosquitoes, blackflies, synanthropic flies,
intermediate-host snails and cockroaches. Most effort has been directed
against mosquitoes because of the human disease agents they transmit.
Consequently, must of this section is devoted to mosquitoes.
The successful widespread use of biological control agents against mosquitoes
will require a much better understanding of the ecology of predator/prey and
pathogen/host relationships (Service 1983). The opportunistic characteristics
of many species (i.e., their ability to exploit temporary habitats, coupled
with their short generation time, high natural mortality, great dispersal
potential, and other R-strategist characteristics) pose difficult problems
for any biological control agent. Mosquitoes typically exploit many aquatic
habitats. Often a biological control agent will have a much narrower range of
environmental activity than the target species. Thus, in many situations a
number of different biological control agents and/or appropriate methods will
be necessary to control even one species of mosquito across its range of
exploitable breeding sources. Fish.--Several
species of fishes are used for the biological control of mosquitoes, and these
species together form the major successes in biological control.
Unfortunately, their usefulness is limited to more permanent bodies of water,
and even under these situations their impact on the target species has been
only partially successful. Bay et al (1976) point out that many species of
fish consume mosquito larvae, but only a few species have been manipulated to
manage mosquito populations.
The mosquitofish, G. affinis, is the best known
agent for mosquito control. This fish, which is native to the southeastern
United States, eastern Mexico and the Caribbean area, was first used as an
introduced agent for mosquito control when it was transported from North
Carolina to New Jersey in 1905 (Lloyd 1987). Shortly thereafter it was
introduced to the Hawaiian Islands to control mosquitoes which had been
introduced during the 19th century. During the next 70 years, the
mosquitofish was transported to over 50 countries and today stands as the
most widely disseminated biological control agent (Bay 1969, Garcia &
Legner 1999, Lloyd 1987). Many of these introductions were aimed at Anopheles species that were
transmitting malaria. Hackett (1937) described its usefulness in malaria control
programs in Europe. He commented that its effects were not sufficient by
themselves, but that the fish had a definite impact on the suppression of the
disease. Tabibzadeh et al. (1970) reported a rather
extensive release program in Iran and concluded that the fish was an
important component in malaria eradication. Sasa and Kurihara (1981) and
Service (1983) believed that the fish had little impact on the disease and
that most evidence is circumstantial. Gambusia
no longer is recommended by the World Health Organization for malaria control
programs, primarily because of its harmful impact on indigenous species of
fish (Service 1983, Lloyd 1987).
The biological attributes of G.
affinis, namely a high
reproductive capability, high survivorship, small size, omnivorous foraging
in shallow water, relatively high tolerance to variations in temperature,
salinity and organic waste, would seemingly make this species an excellent
biological control agent (Bay et
al. 1976, Moyle 1976).
However, whether this fish leads to effective mosquito control at practical
costs in many situations is still debated. Kligler\'s (1930) statement that
"... their usefulness as larvae-destroyers under local conditions where
vegetation is abundant and micro fauna rich enough to supply their needs
without great trouble, is limited. In moderately clear canals, on the other
hand, or in pools having a limited food supply, they yielded excellent
results ..." is probably one of the most accurate.
In California this fish had been used extensively for control of mosquitoes
in various habitats (Bay et al. 1976). Many mosquito
abatement districts in the State have developed systems for culturing,
harvesting and winter storage of the mosquito fish to have enough available
for planting early in the spring (Coykendall 1980). This is particularly
important in the rice growing areas of California where early stocking
appears to be of critical importance for build-up of fish populations to
control mosquitoes during late summer. The results of the use of G. affinis in California rice fields will be summarized below
as an illustrative example of the mixed successes achieved in the field.
Rice cultivation in California continuously poses one of the most difficult
control problems for Anopheles
and Culex species. Hoy &
Reed (1970) showed that good to very good control of Culex tarsalis
Coquillett could be achieved at stocking rates of about 480 or more females
per hectare, and Stewart et al (1983) reported excellent control with a
similar stocking rate against this species in the San Joaquin Valley.
Although Cx. tarsalis appears to be
controlled effectively by G.
affinis, the control of its
frequent companion in northern California rice fields, Anopheles freeborni
Aitken, is less apparent. Hoy et al. (1971) showed a reduction of An. freeborni populations at various stocking rates of about
120 to 720 fish per hectare, but the reduction was not nearly as striking as
for Cx. tarsalis. These workers surmised that improvement in
control could be achieved by earlier season stocking, possibly multiple
release points in fields and a reliable source of healthy fish for stocking.
Despite an extensive research effort in mass culture, management and storage
for G. affinis by the State of California (Hoy & Reed 1971),
a mass production method has not been satisfactorily achieved (Downs et al. 1986, Cech and Linden 1987).
Studies of G. affinis for control of
mosquitoes in wild rice show that relatively high stocking rates can
effectively reduce An. freeborni and Cx. tarsalis populations within a three-month period (Kramer et al. 1987a). The commercial production of wild rice, which
is a more robust and toller plant than white rice and requires only 90
instead of 150 days to mature, has been increasing over the last few years in
California (Kramer et al. 1987). In the above study,
stocking rates of 1.7 Kg/ha (ca. 2400 fish/Kg) released in 1/10 ha wild rice
plots failed to show a significant difference in reduction of mosquitoes from
plots with no fish. A decrease in numbers of larvae was noted just prior to
harvest which suggested that the fish were beginning to have an impact on
mosquito numbers (Kramer et al. 1987). Numbers of fish in
these plots, based on recovery after drainage, was about 100,000 individuals
per hectare (ca. 32 Kg/ha) or a density of about 10 fish per square meter.
However, significant control was not achieved.
During 1987 this study was repeated at the rates of 1.7 and 3.4 Kg/ha of
fish. Results showed an average suppression of larvae (primarily An. freeborni) of <1 and 0.5 per dip for the low and
high rate respectively, compared to control plots which averaged >4.5 per
dip. Fish densities in the 1987 study surpassed those of 1986 by about two
fold at the 1.7 Kg/ha rate and three fold at the 3.4 Kg/ha rate. It is
believed that these greater fish numbers accounted for the control
differences observed in the second year, although mosquitoes were not
eliminated. Differences between test plots and control plots were first
observed eight weeks after the fish had been planted and mosquitoes remained
under control until drainage of the fields (Kramer et al.
1988).
Davey & Meisch (1977a,b) showed that the mosquitofish at inundative
release rates of 4,800 fish per hectare, was effective for control of Psorophora columbiae (Dyar & Knab) in Arkansas rice fields. Fish
released at the water flow inlets dispersed quickly throughout the fields.
This is an important attribute for controlling species of Psorophora and Aedes, whose hatch and larval
development are completed within a few days. A combination of 1,200 G. affinis and about 300 sunfish (Lepomis cyanellus
Rafinesque) gave better control than either four times the amount of G. affinis or L.
cyanellus used separately.
This synergistic effect reduces logistic problems associated with having
enough fish available at the times fields are inundated. Blaustein (1986)
found enhanced control of An.
freeborni by mosquitofish in
California rice fields after the addition of green sunfish. He speculated
that the increased control was the result of the mosquitofish spending more
time in protected areas where mosquitoes were more abundant and the green
sunfish was avoided. The availability of fish for stocking fields either
inundatively, such as in Arkansas or for control later in the season as
practiced in California, has been a fundamental reason why fish have not been
used more extensively in rice fields.
A unique use of the mosquitofish by inundative release was reported by Farley
& Caton (1982). The fish were released in subterranean urban storm drains
to control Culex quinquefasciatus Say breeding
in entrapped water at low points in the system. Fish releases were made
following the last major rains to avoid having them flushed out of the
system. Fish survived for more than three months during the summer and were
found throughout the system. Gravid females produced progeny. However, no
mating occurred, and after the initial increase in numbers populations of
fish diminished as summer progressed. Reductions of mosquitoes from 75 to 94%
were observed for three months compared to untreated areas (Mulligan et al. 1983). This control practice is now conducted on a
routine basis by the Fresno Mosquito Abatement District (J. R. Caton 1987,
pers. comm.).
Although G. affinis has been useful for
control of mosquitoes in a number of situations, clearly there are drawbacks
to its use. In fact, if today\'s environmental awareness existed at the turn
of the century, this fish probably never would have been intentionally
introduced into exotic areas (Pelzman 1975, Lloyd 1987). The major objection
to this fish has been its direct impact on native fishes through predation,
or its indirect impact through competition (Bay et al.
1976, Schoenherr 1981, Lloyd 1987). More than 30 species of native fish have
been adversely affected by the introduction of Gambusia (Schoenherr 1981, Lloyd 1987). Gambusia, a general predator,
can also substantially reduce zooplankton and thus lead to algal blooms in
certain situations (Hurlbert et
al. 1972). Introductions of Gambusia have also reduced
numbers of other aquatic invertebrates coinhabiting the same waters (Hoy et al. 1972, Farley & Younce 1977, Rees 1979, Walters
& Legner 1980, Hurlbert & Mulla 1981).
The next most widely used fish for mosquito control is the common guppy, Poecilia reticulata (Peters). It has been deployed successfully in
Asia for the control of waste water mosquitoes, especially Cx. quinquefasciatus. Like its poeciliid relative Gambusia, it is native to the
Americas (tropical South America). But, rather than being intentionally
introduced to control mosquitoes, it was taken to other parts of the world by
tropical fish fanciers. Sasa et al. (1965) observed wild populations of this
fish breeding in drains in Bangkok and concluded from their observations that
it was controlling mosquitoes common to that habitat. The practical use of
guppies is primarily restricted to subtropical climates because of an
inability to tolerate temperate-zone water temperatures (Sasa & Kurihara
1981). However, their most important attribute is a tolerance to relatively
high levels of organic pollutants, which makes them ideal for urban water
sources that are rich in organic wastes. In Sri Lanka, wild populations have
been harvested and used for the control of mosquitoes in abandoned wells,
coconut husk pits and other sources rich in organics (Sasa & Kurihara
1981). The fish occursin in India, Indonesia and China and has been
intentionally introduced for filariasis control into Burma (Sasa &
Kurihara 1981). Mian et al (1985) evaluated its use for control of mosquitoes
in sewage treatment facilities in southern California and concluded that
guppies showed great potential for mosquito control in these situations.
Exotic fish have also been used for clearing aquatic vegetation from
waterways which has resulted in excellent mosquito control. In the irrigation
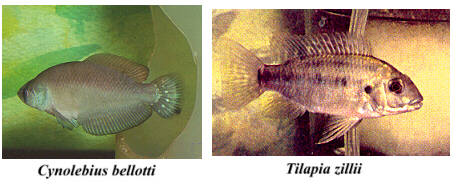
systems of southeastern California, three species of subtropical cichlids, Tilapia zillii (Gervais), Oreochromis
(Sarotherodon) mossambica
(Peters), and Oreochromis
(Sarotherodon) hornorum
(Trewazas), were introduced and have become established over some 2,000 ha of
Cx. tarsalis breeding habitat (Legner & Sjogren 1984). In
this situation, mosquito populations are under control by a combination of
direct predation and the consumption of aquatic plants by these omnivorous
fishes (Legner & Medved 1973, Legner 1978a, 1983; Legner & Fisher
1980; Legner & Murray 1981, Legner & Pelsue 1983). As Legner &
Sjogren (1984) indicate, this is a unique example of persistent biological
control and probably only applicable for relatively sophisticated irrigations
systems where a permanent water supply is assured, and water conditions are
suitable to support the fish (Legner et
al. 1980). There is a
three-fold advantage in the use of these fish: (1) clearing of vegetation to
keep waterways open, (2) mosquito control and (3) a fish large enough to be
caught for human consumption. Some sophistication is necessary when stocking
these cichlids for aquatic weed control, which is often not understood by
irrigation districts personnel (Hauser et
al. 1976, 1977; Legner
1978b). Otherwise competitive displacement may eliminate T. zillii,
the most efficient weed eating species (Legner 1986).
Household storage of water in open containers has frequently been the cause
for outbreaks of human disease transmitted by Aedes aegypti
(Linnaeus) in less developed parts of the world. While conducting Ae. aegypti surveys in Malaysia during the mid 1960s, Dr.
Richard Garcia , of UC Berkeley observed what were apparently P. reticulata being utilized by town residents for the
control of mosquitoes in bath and drinking water storage containers. The
origin of this control technique was not clear but it appeared to be a custom
brought to the area by Chinese immigrants. Not all residents used fish, but
those that did had no breeding populations of Ae. aegypti.
Neng (1987) reported on the use of a catfish, Claris sp., for the control of Ae. aegypti
in water storage tanks in coastal villages of southern China. This fish was
considered appropriate since it was indigenous, edible, consumed large
numbers of mosquito larvae, had a high tolerance for adverse conditions and could be obtained from the local markets.
One fish was placed in each water source and later checked for its presence
by larval survey teams about every 10 to 15 days. If fish were not found on
inspection the occupant was told to replace the fish or be fined. The
investigation was conducted from 1981 to 1985, and surveys over this period
showed a sharp initial reduction in Ae.
aegypti followed by a low
occurrence of the mosquito over the four-year study period. Outbreaks of
dengue were observed in neighboring provinces during this period, but not in
the fishing villages under observation. The cost of the program was estimated
to be about 1/15 that of indoor house spraying (Neng 1987).
Alio et al. (1985) described another use of a local
species of fish for the control of a malaria vector similar to the method
reported by Kligler (1930). Oreochromis
sp., a tilapine, was introduced into human-made water catchment basins called
"barkits" in the semi arid region of northern Somalia. These small
scattered impoundments served as the only sources of water during the dry
season for the large pastoral population of the area. Anopheles arabiensis
Patton, the vector of malaria in that area, is essentially restricted to
these sites. Release of fish into the "barkits" dramatically
reduced both the vector and nonvector populations of mosquitoes rather
quickly. Treatment of the human population with antimalarial drugs during the
initial phase of this two-year study, combined with the lower vector
population reduced the transmission rate of malaria to insignificance over a
21 month period whereas the control villages remained above 10 percent. Alio
et al (1985) commented that the added benefits of reduced vegetation and
insects in the water sources was also recognized by the local population.
This resulted in community cooperation and was expected to further benefit
the control strategy by providing assistance in fish distribution and
maintenance as the program expanded to other areas.
The last two examples involve the use of indigenous over exotic fish where
feasible in vector control programs. There are other examples where native
fishes have been used in specialized circumstances (Kligler 1930, Legner et al. 1974, Menon & Rajagopalan 1978, Walters &
Legner 1980, Ataur-Rahim 1981 and Luh 1981). Lloyd (1987) argued that only
indigenous fish should be employed for mosquito control because of the
environmental disruption induced by exotics such as G. affinis.
However, he suggested that native fish should be analyzed carefully for prey
selectivity, reproductive potential and effectiveness in suppression pest
populations before attempting their use. Lloyd (1987) also pointed out that a
multidisciplinary approach involving fisheries biologists and entomologists
should be employed when developing indigenous fish for mosquito control.
However, in California where native pup fishes in the genus Cyprinodon may afford a greater
potential for mosquito control under a wider range of environmental stresses
than Gambusia (Walters &
Legner 1980), the California Department of Fish and Game discourages their
use on the basis that unknown harmful effects might result to other
indigenous fishes. There is also the concern that certain rare species of Cyprinodon might be lost
through hybridization.
Perhaps China\'s example of a multipurpose use of native fish for mosquito
control and a human protein source is the most resourceful strategy. This
application for mosquito control is not new. Kligler (1930) used a tilapine
fish to control Anopheles
sp. in citrus irrigation systems in old Palestine, where farmers cared for
the fish, consuming the larger ones. According to Luh (1981), the culture of
edible fish for the purpose of mosquito control and human food is not widely
encouraged in China. The old Chinese peasant custom of raising edible fish in
rice fields has received greater attention in recent times because of the
benefits made possible through this practice. The common carp, Cyprinus carpio Linnaeus, and the grass carp, Ctenopharygodon idella
Valenciennes, are most commonly used. Fish are released as fry at the time
rice seedlings are planted. Fields are specially prepared with a central
"fish pit" and radiating ditches for refuge when water levels are
low. Pisciculture in rice fields, as noted by Luh (1981), has three major
benefits: (1) a significant reduction in culicine and to a lesser extent
anopheline larvae, (2) fish are harvested as food and (3) rice yields are
increased apparently by a reduction in competitors and possibly by
fertilization of the plants by fish excreta.
Another group of fishes, the so-called "instant" or annual
fishes, (Cyprinodontidae), which are native to South America and
Africa, have been considered as possible biological control agents for
mosquitoes (Vanderplant 1941, 1967; Hildemann & Wolford 1963; Bay 1965,
1972; Markofsky & Matias 1979). The relatively drought resistant eggs of
these cyprinodontids, which allows them to utilize temporary water sources as
habitat, would seem to make them ideal candidates for mosquito control. There
is also some evidence that they do impact mosquito populations in native
areas (Vanderplant 1941, Hildemann & Wolford 1963, Markofsky & Matias
1979). Research on the biology and ecology of several species has been
conducted; however, there are no published accounts on the successful use of
these fish in field situations. In California the South American species Cynolebias nigripinnis Regan and Cynolebias
bellottii (Steindachner),
survived the summer in rice fields, but no reproduction was observed over a
three-year period (Coykendall 1980). It was speculated that they may play a
future role in California\'s mosquito control program in temporary pools and
possibly rice fields. C. bellottii was observed to
reproduce repeatedly and to persist in small intermittently dried ponds in
Riverside, California for eleven consecutive years, 1968-1979 (Legner &
Walters unpubl.). Four drying flooding operations over two months were
required to eliminate this species from ponds that were to be used for native
fish studies (Walters & Legner 1980). It seems logical, given the biological
capability of surviving an annual dry period, that these fish could be
successfully integrated into mosquito control programs, especially in newly
created sources in geographic areas where they naturally occur (Vaz-Ferreira et al. 1963, Anon 1981, and Geberich 1985). Arthropods.--Numerous species of predatory
arthropods have been observed preying on mosquitoes, and in some cases are
believed to be important in controlling mosquitos (James 1964, Service 1977,
Collins & Washino 1979, McDonald & Buchanan 1981). However, among the
several hundred predatory species observed, only a few have been used in a
manipulative way to control mosquitoes. Dragonflies, sometimes referred to as
mosquito hawks, were one of the first arthropods to be examined. Difficulties
in colonization, production and handling have restricted their use to
experimental observation. It is unlikely that they will ever be used
extensively (Lamborn 1890, Beesley 1974, El Rayah 1975, Riviere et al. 1987a).
There are a few cases where the difficulties associated with the manipulative
use of arthropods has been at least partly overcome. More than 50 years ago,
in a classic use of biological control, the mosquito Toxorhynchites, whose larvae are predators of other
mosquitoes, was released on several Pacific Islands in an effort to control
natural and artificial container breeding mosquitoes such as Ae. aegypti and Aedes
albopictus (Skuse) (Paine
1934, Bonnet & Hu 1951, Petersen 1956). The releases were not considered
successful, but the mosquitoes did establish in some areas (Steffan 1975).
Several reasons to explain why these releases failed were low egg production,
lack of synchrony between predator and prey life cycles, and selection of
only a relatively small number of prey breeding sites (Muspratt 1951,
Nakagawa 1963, Trpis 1973, Bay 1974, Riviere 1985).
Although not apparently a suitable predator in the classical sense, there is
still interest in the use of various Toxorhynchites
spp. for inundative release (Gerbert & Visser 1978). Trpis (1981) working
with Toxorhynchites brevipalpis (Theobald) showed
that the high daily consumption rate and long survival of the larvae without
prey made it a prime candidate for biological control use. Observations on
adult females indicated a 50% survivorship over a 10-week period with a
relatively high oviposition rate per female. All the above attributes suggest
that this species would be useful for inundative release programs against
container breeding mosquitoes. Studies by Focks et al (1979) in Florida,
working with Toxorhynchites rutilis rutilis Coquillett, showed that this species had a high
success rate in artificial breeding containers. In a 12.6 hectare residential
area, about 70% of the available oviposition sites were located over a 14-day
period by two releases of 175 females. Mass culturing techniques have been
developed for this species and Toxorhynchites
amboinensis (Doleschall)
(Focks & Boston 1979, Riviere et
al. 1987b).
Focks et al (1986), working with Toxorhynchites
amboinensis, reported that
release of 100 females per block for several weeks, combined with ultra low
volume application of malathion, reduced Ae.
aegypti populations by about
96% in a residential area of New Orleans. The Toxorhynchites releases and not the insecticide treatment
apparently accounted for most of the reduction. These workers noted that the
procedure could be further refined by reducing both the number of predators
and malathion applications without lowering efficacy. Mosquitoes such as Ae. aegypti and Ae.
albopictus, which breed in
and whose eggs are dispersed via artificial containers, pose major health
hazards as vectors of human diseases throughout much of the warmer climates
of the world. The massive quantities of containerized products and rubber
tires which are then discarded without care or stockpiled, have given these
mosquito species a tremendous ecological advantage. The recent establishment
and extensive spread of Ae. albopictus in the United States
underlines this point (Sprenger & Wuithironyagool 1986). The apparent
inability of governments to appropriately control disposal of these
containers and difficulties in location once they are discarded makes
inundative releases of Toxorhynchites,
either alone or in combination with other control tactics, a much more
plausible approach (Focks et
al. 1986, Riviere et al. 1987a).
Notonectids are voracious predators of mosquito larvae under experimental
conditions (Ellis & Borden 1970, Garcia et al.
1974, Hazelrig 1974), and in waterfowl refuges in California\'s Central
Valley (Legner & Sjogren, unpub. data). Notonecta undulata
Say and Notonecta unifasciata Guerin have been
colonized in the laboratory. In addition, collection of large numbers of
eggs, nymphs and adults is feasible from such breeding sites as sewage
oxidation ponds (Ellis & Borden 1969, Garcia 1973, Hazelrig 1975, Sjogren
& Legner 1974, Muira 1986). Some studies have been conducted on storage
of eggs at low temperatures, but viability decreased rapidly with time (Sjogren
& Legner 1989). At present, the most feasible use of these predators
appears to lie in the recovery of eggs from wild populations on artificial
oviposition materials and their redistribution to mosquito breeding sites.
Such investigations were carried out in central California rice fields by
Miura (1986). Floating vegetation such as algal mats and sometimes duck weed
(Lemna spp.) form protective
refugia for mosquito larvae, and consequently populations of mosquitoes can
be high in the presence of notonectids (Garcia et al.
1974). It appears that colonization and mass production costs, coupled with
the logistics of distribution, handling and timing of release at the
appropriate breeding site, are almost insurmountable problems for routine use
of notonectids in mosquito control.
In addition to insect predators, several crustaceans feed on mosquito larvae.
Among these are the tadpole shrimp, Triops longicaudatus
(LeConte), and several copepod species. Mulla et al. (1986)
and Tietze & Mulla (1987), investigating the tadpole shrimp, showed that
it was an effective predator under laboratory conditions and speculated that
it may play an important role in the field against flood water Aedes and Psorophora species in southern California. Drought
resistance in predator eggs is an appealing attribute for egg production,
storage and manipulationin field situations against these mosquitoes.
However, synchrony in hatch and development between the predator and the prey
is crucial if this is to be a successful biological control agent for the
rapidly developing Aedes and
Psorophora spp. In addition,
the tadpole shrimp is considered an important pest in commercial rice fields.
Miura & Takahashi (1985) reported that Cyclops vernalis
Fisher was an effective predator on early instar Cx. tarsalis
larvae in the laboratory. These workers speculated that copepods could have
an important role in suppressing mosquito populations in rice fields because
of their feeding behavior and abundance.
Another crustacean that has shown promise for more extensive application is
the cyclopoid predator, Mesocyclops
aspericornis Daday (Riviere et al. 1987b). This work has shown reductions of Ae. aegypti and Ae.
polynesiensis Marks by more
than 90% after inoculative release of the organism into artificial
containers, wells, treeholes and land crab burrows. Although not able to
withstand desiccation, the rather small cyclopod predator has persisted
almost 2.5 years in crabholes and up to five years in wells, tires and
treeholes under subtropical conditions. This species can be mass produced,
but its occurrence in large numbers in local water sources allows for the
inexpensive and widespread application to mosquito breeding sites in
Polynesia (Riviere et al. 1987a,b). The species is
also very tolerant of salinities greater than 50 parts per thousand. The
benthic feeding behavior of Mesocyclops
makes it an effective predator of the bottom foraging Aedes, but limits effectiveness against surface foraging
mosquitoes. Riviere et al. (1987a,b) believed that the
effectiveness against Aedes
is due to a combination of predation and competition for food. Perhaps the
greatest utility of this Mesocyclops
will lie in the control of crabhole breeding species, such as Ae. polynesiensis in the South Pacific. Further investigations
may uncover additional cyclopods that can impact other mosquito species.
The most important nonarthropod invertebrate predators to draw attention for
mosquito control are the turbellarian
flatworms and a coelenterate. Several flatworm species have been shown
to be excellent predators of mosquito larvae in a variety of aquatic habitats
(Legner & Medved 1974, Yu & Legner 1976, Collins & Washino 1978,
Case & Washino 1979, Legner 1977, 1979, Ali & Mulla 1983, George et al. 1983). Several biological and ecological attributes of
flatworms would seem to make them ideal candidates for manipulative use.
Among them are ease of mass production, an overwintering embryo, effective
predatory behavior in shallow waters with emergent vegetation, on site
exponential reproduction following inoculation (Medved & Legner 1974,
Tsai & Legner 1977, Legner & Tsai 1978, Legner 1979) and tolerance to
environmental contaminants (Levy & Miller 1978, Nelson 1979).
Collins & Washino (1978) and Case & Washino (1979) suggested that
flatworms, particularly Mesostoma,
may play an important role in the natural regulation of mosquitoes in some
California rice fields because of their densities and their predatory attack
on mosquito larvae in sentinel cages. Preliminary analysis using extensive
sampling showed a significant negative correlation between the presence of
flatworms and population levels of Cx.
tarsalis and An. freeborni (Case & Washino 1979). However, these
workers cautioned that an alternative hypothesis related to the ecology of
these species may have accounted for the correlations. Later investigations
by Palchick & Washino (1984), employing more restrictive sampling, were
not able to confirm the correlations between Mesostoma and mosquito populations. However, the enormity
of the problem associated with sampling in California rice fields, coupled
with the complexity of the prey and predator interactions, make further
studies necessary before the role of this group of flatworms in rice fields
can be clearly established.
The important attributes for manipulative use of flatworms mentioned above
raises the question of why they have not been developed further for use in
mosquito control. Perhaps the contemporary development of Bacillus thuringiensis var. israelensis
DeBarjac (H-14), a highly selective easily applied microbial insecticide, may
have been at least partially responsible for slowing further work and development
of these predators. Their mass culture must be continuous and demands skilled
technical assistants (Legner & Tsai 1978). Their persistence in field
habitats may also depend on the presence of other organisms, such as
ostracods, which can be utilized for food during low mosquito abundance
(Legner et al. 1976).
The coelenterates, like the flatworms, showed great promise for further
development and use in selected breeding habitats. Chlorohydra viridissima
(Pallas) is efficient in suppressing culicine larvae in ponds with dense
vegetation and this species also can be mass produced (Lenhoff & Brown
1970, Yu et al. 1974a,b, 1975). However,
like the flatworms, work on these predators has waned, perhaps for similar
reasons as speculated for the flatworms. Microbial pesticides can be employed
over an extensive range of different mosquito breeding habitats. Also,
commercial production of flatworms and coelenterates would be much more
costly, and storage of viable cultures all but impossible. Fungi.--The most promising fungal pathogen is
a highly selective and environmentally safe oomycete, Lagenidium giganteum
Couch. First tested for its pathogenicity to mosquitoes in the field by
McCray et al. (1973), it is applied by
aircraft to rice fields (Kerwin & Washino 1987). Lagenidium develops asexually and sexually in mosquito
larvae, and is capable of recycling in standing bodies of water. This creates
the potential for prolonged infection in overlapping generations of
mosquitoes. Lagenidium may
also remain dormant after the water source has dried up and then become
active again when water returns. The sexually produced oospore offers the
most promising stage for commercial production because of its resistance to
desiccation and long-term stability. However, problems in production and
activation of the oospores still remain (Axtell et al.
1982, Merriam & Axtell 1982a,b, 1983; Jaronski & Axtell 1983a,b,c,
Kerwin et al. 1986, Kerwin & Washino
1987). Field trials with the sexual oospore and the asexual zoospore indicate
that this mosquito pathogen is near the goal of practical utilization. Kerwin
et al (1986) reports that the asynchronous germination of the oospore is of
particular advantage in breeding sources where larval populations of
mosquitoes are relatively low, but recruitment of mosquitoes is continuous
due to successive and overlapping generations, as in California rice fields.
The germination of oospores over several months provides long-term control
for these continuous low level populations. In addition, the asexual
zoospores arising from the oospore infected mosquito is available every two
to three days to respond in a density dependent manner to suppress any
resurging mosquito population. This stage survives about 48 hours after
emerging from the infected host.
Kerwin et al. (1986) indicate that laboratory
fermentation production of the asexual stage of Lagenidium for controlling mosquitoes in the field is
approaching the development requirements and costs for the production of Bacillus thuringiensis israelensis.
A distinct advantage of this pathogen over the Bacillus is its potential to recycle through successive
host generations. The disadvantage of the asexual stage is that it is
relatively fragile, cannot be dried and has a maximum storage life of only
eight weeks (Kerwin & Washino 1987). Thus, the focus of attention for
commercial production is on the oospore, which is resistant to desiccation
and can be easily stored. Axtell & Guzman (1987) have recently
encapsulated both the sexual and asexual stages in calcium alginate and
reported activity against mosquito larvae after storage for up to 35 and 75
days, respectively. Further refinement in techniques of production and
encapsulation might make this approach a viable option for future commercial
production and application.
Limitations on the use of this pathogen include intolerance to polluted
water, salinity and other environmental factors (Jaronski & Axtell 1982,
Lord & Roberts 1985, Kerwin & Washino 1987). However, there are numerous
mosquito breeding sources where these limitations do not exist and therefore
one would expect to see this selective and persistent pathogen available for
routine mosquito control in the near future.
The fungus Culicinomyces clavosporus Couch, Romney &
Rao, first isolated from laboratory mosquito colonies and later from field
habitats, has been under research and development for more than a decade
(Sweeney et al. 1973, Couch et al. 1974, Russell et
al. 1979, Frances et al. 1985). The fungus is active against a wide range of
mosquito species and also causes infections in other aquatic Diptera (Knight
1980, Sweeney 1981). The ease of production with relatively inexpensive media
in fermentation tanks is an extremely desirable trait. However, problems in
storage must be overcome if this fungus is to be widely used. Perhaps a
drying process, now being investigated, will solve storage requirements
(Sweeney 1987). Although the fungus has shown high infection rates in field
trials, dosage rates have been high and appreciable persistence at the site
has not been demonstrated (Sweeney et
al. 1973, Lacey & Undeen
1986, Sweeney 1983, 1987).
Various species of Coelomomyces
have been studied over the last two decades for use in mosquito control.
Natural epizootics with infection rates in excess of 90% have been recorded.
These fungi persist in certain habitats for long periods; however, factors
triggering outbreaks in these situations are not well understood (Chapman
1974). Some field testing has been done, but results have been highly
variable (Federici 1981). In general, difficulties associated with the
complex life cycle of these fungi have encumbered research on them. Federici
(1981) and Lacey & Undeen (1986) have reviewed the potential of these
fungi for mosquito control. Nematodes.--Among the various nematodes pathogenic
for mosquitoes, Romanomermis
culicivorax Ross &
Smith, has received the most attention (Petersen & Willis 1970, 1972a,b,
1975; Brown et al. 1977, Brown & Platzer
1977, Poinar 1979, Petersen 1980a,b, Brown-Westerdahl et al.
1982, Kerwin & Washino 1984). This mermithid, which is active against a
wide range of mosquito species, has been mass produced (Petersen & Willis
1972a) and utilized in a number of field trials. The nematode was
commercially produced and sold under the name Skeeter Doom TMR,
but according to Service (1983) eggs showed reduced viability in transport
and the product currently is no longer sold. However, the nematode\'s ability
to recycle through multigenerations of mosquitoes and overwinter in various
habitats, including drained, harvested, stubble-burned, cultivated and
replanted rice fields, are strong attributes favoring its further research
and development for biological control (Petersen & Willis 1975,
Brown-Westerdahl et al. 1982). Several field
applications have shown good results and have included both the preparasitic
stage and post parasitic stages with the former more applicable to the
"quick kill" and the latter for more long-term continuous control
such as in California rice fields (Petersen et al.
1978a,b, Levy et al. 1979, Brown-Westerdahl et al. 1982). Some drawbacks to its widespread use include
intolerance to low levels of salinity, polluted water and low oxygen levels,
predation by aquatic organisms and the potential for development of
resistance by the host (Petersen & Willis 1970, Brown & Platzer 1977,
Brown et al. 1977, Petersen 1978,
Brown-Westerdahl 1982). However, these environmental problems are not
generally an issue for anopheline control. For control of these species the
cost of in vivo mass production clearly
stands as the major drawback for this pathogen. Perhaps its most plausible
use will be in specialized habitats integrated with other control strategies
(Brown-Westerdahl et al. 1982). Bacteria.--The spore forming bacterial pathogen, Bacillus thuringiensis var. israelensis
(H-14), was isolated by Goldberg & Margalit (1977) and the produced toxin
has been shown by numerous studies to be an effective and environmentally
sound microbial insecticide against mosquitoes and blackflies. Its high
degree of specificity and toxicity, coupled with its relative ease of
production, have made it the most widely used microbial product to date for
mosquito and blackfly control. Several formulations are currently available
from commercial firms throughout the world. Its efficacy under different
environmental conditions and problems associated with its use have been
reviewed by Garcia (1986, 1987) and Lacey & Undeen (1986).
Another spore forming bacterium, Bacillus
sphaericus Neide, has also
shown great promise as a larvacide against certain mosquito species (Mulla et al. 1984). In general, several strains of this pathogen
show a much higher degree of toxic variability among species of mosquitoes. Culex spp. appear to be highly
susceptible, whereas other species such as Ae. aegypti
are highly refractory. Unlike the ephemeral larvacidal activity of Bacillus t. i.
toxin, some strains of B. sphaericus have shown
persistence and apparent recycling in certain aquatic habitats (DesRochers
& Garcia 1984). For further detail see the recent review by Lacey &
Undeen (1986). Protozoa.--A large number of protozoa have been
isolated from mosquitoes and other medically important arthropods (Roberts et al. 1983, Lacey & Undeen 1986). Of this assemblage the
microsporidians have been
studied rather intensively. Due to their complex life cycle and the in vivo production methods necessary for maintaining them,
research on their practical utility has been limited. However, as Lacey &
Undeen (1986) point out, if more information is developed on their life
cycle, it may be found that they could play a role in suppressing mosquitoes
through inoculative and augmentive releases in certain habitats.
Among the other protozoa that show promise is the endoparasitic ciliate, Lambornella clarki Corliss & Coats, a
natural pathogen of the treehole mosquito, Aedes sierrensis
Ludlow. This pathogen has received considerable attention over the last few
years as a potential biological control agent for container breeding
mosquitoes (Egeter et al. 1986, Washburn &
Anderson 1986). Desiccation resistant cysts allow persistence of the ciliate
from one year to the next. Currently, in
vitro production methods are
being developed and small field trials are being initiated to determine its
efficacy and practicability for field use (Anderson et al.
1986a,b). Viruses.--Numerous pathogenic viruses have been
isolated from mosquitoes and blackflies. However, to date none look promising
for practical use in control (Lacey & Undeen 1986). SYNANTHROPIC DIPTERA These
flies, the most important of which are muscoid species, can be defined
broadly as those most closely associated with human activities. Breeding
habitats very from the organic wastes of urban and rural settlements to those
provided by various agricultural practices, particularly ones related to the
management and care of domestic and range animals. Their degree of
relationship to humans varies considerably with the ecology and behavior of
the fly involved. Some are more often found inside dwellings (endophilic) while others remain
mostly outdoors (exophilic).
The discussion that follows separates these flies by their general endophilic
and exophilic habits, and is restricted to brief comments since the potential
for biological control of these flies has been recently reviewed (Legner et al. 1974, Bay et
al. 1976, Legner 1986).
Endophilic Flies.--Povolny
(1971) describes these flies as primarily dependent on human and domestic
animal wastes. Musca domestica Linnaeus is by far
the best known example. However, some Drosophila
and Psychoda spp. also fall
into this category. Certain Fannia
spp. are more on the periphery but are also included here.
The common housefly, Musca domestica, has been a constant
associate of humans over much of our modern history. Attempts to control its populations
by biological means have been extensive and on occasion successful in special
situations. More frequently they have failed to reduce numbers to acceptable
levels. It should be emphasized that control of M. domestica
populations, as well as most other endophilic flies pestiferous to humans,
would be largely unnecessary if waste products produced by human activities
could be appropriately managed. Since this is not the case, efforts towards
the biological control of these species have continued.
Starting around the turn of this century biological control of these flies
was attempted by the introduction of a broad range of different natural
enemies into areas where the flies presented problems. The Pacific Islands were
a focus of much attention with the introduction of dung beetles, several
parasitoids and predators during this period. It was believed that the
accidental introduction of an ant, Pheidole
megacephala F., combined
with the introduction of the coprophagous dung beetle Hister chinensis
Quensel, caused significant fly reductions on the islands of Fiji and Samoa
(Simmonds 1958). The Islands of Hawaii had 16 introductions from 1909 to 1967
of which 12 established. However, the exact role of these natural enemies in
overall regulation of flies on the islands is still not well understood
(Legner et al. 1974, Legner 1978c).
Rodriguez & Riehl (1962) in California, used the novel and successful
approach of chicken cockerels
as direct predators of fly larvae in chicken and rabbit manure. However, this
technique is utilized very little today because of the threat that roving
birds pose to the spread of avian pathogens.
Research over the last two decades has centered on the more highly
destructive parasitoid and predatory species. Examples, such as the encyrtid Tachinaephagus zealandicus Ashmead, five
species of the pteromalid genus Muscidifurax,
and Spalangia sp. were
evaluated for their capabilities of attacking dipterous larvae and pupae in
various breeding sources. They are believed to be capable of successful fly
suppression if the right species and strains are applied in the right
locality (Legner & Brydon 1966, Legner & Dietrick 1972, 1974; Morgan et al. 1975, 1977; Olton & Legner 1975, Pickens et al. 1975, Morgan & Patterson 1977, Rutz & Axtell
1979, Propp & Morgan 1985, Axtell & Rutz 1986, Legner 1988b,
Mandeville et al. 1988, Pawson & Petersen
1988). Other approaches have included the use of pathogens and predatory
mites, and inundative releases of parasitoids and predators (Ripa 1986).
Although partially successful, none of these strategies have become the sole
method for fly control, and the wrong choice of a parasitoid strain may have
detrimental results (Legner 1988b). Instead, the focus is on integrated
controls including other methods such as cultural, adult baiting and aerosol
treatments with short residual insecticides. However, it is generally agreed
that existing predatory complexes exert great influences on fly densities
(Legner et al. 1975b, 1980; Geden 1984,
Geden et al. 1987, 1988; Geden &
Axtell 1988) and that many biological control agents of endophilous flies
have not been thoroughly surveyed, nor their potential adequately assessed
(Mullens 1986, Mullens et al. 1986).
Exophilic Flies.--These
species include flies that
persist in nature in the absence of humans, but whose populations can
increase dramatically as a result of certain human activities such as
providing more breeding habitat. They include several species in the genera Calliphora, Hippelates, Musca, Muscina, Phaenicia,
Stomoxys.
Some success has been recorded with the use of natural enemies against the
calliphorid species in California and Hawaii, but attempts elsewhere in the
world have not been effective (Bay et
al. 1976). The braconid
parasitoid Alysia ridibunda Say, indigenous to
parts of the United States, was released into an area of Texas new to its
range and successfully parasitized the blowflies Phaenicia sericata
(Meigen), and a Sarcophaga
species. However, the parasitoid did not maintain control and became rare
within a couple of years (Lindquist 1940).
The gregarious parasitoid T.
zealandicus may have
considerable potential for biological control of exophilic flies (Olton &
Legner 1975). The range of habitats utilized by this natural enemy is
considered unparalleled by any other fly parasitoid. However, extensive work
with this genus, from the standpoint of field use, has not been given the
this genus has not been given much attention. But one species, Tachinaephagus stomoxcida Subba-Rao, provides
overall permanent reductions of Stomoxys
in Mauritius (Greathead & Monty 1982).
The complex of problems that confront field programs in biological control of
exophilic flies has clearly had a dampening effect on research in this area.
The unforseen problems associated with attempts to biologically control the
eye gnat, Hippelates collusor (Townsend), in
California exemplify those problems. In the early 1960s, a concerted effort
was mounted to control this gnat with the use of both indigenous and exotic
parasitoids in orchards in southern California. About a dozen species and
strains were evaluated for several years. Some of the exotics established,
but eye gnat reductions were obvious only where cultivation practices were
curtailed (Legner et al. 1966, Legner 1970).
Cultivation of the orchards buried the larvae and pupae of the eye gnat below
the search zone of the parasitoids and cultivation also removed vegetation
that offered the parasitoids protection and possibly nutrients (Legner 1968,
Legner & Olton 1969, Legner & Bay 1970). Buried eye gnats emerged
from several centimeters below the soil surface and thus continued to pose a
serious problem (Bay et al. 1976).
The recent discovery of a group of genes, called wary genes, in parasitoids of synanthropic Diptera affords
greater opportunities for biological control (Legner 1987, 19898a, 1989).
Inheritance of quantitative behavior associated with gregarious oviposition
and fecundity in the South American parasitoid Muscidifurax raptorellus
Kogan & Legner (Kogan & Legner 1970) is accompanied by unique
extranuclear influences which cause changes in the oviposition phenotypes of
females prior to the production of their progeny (Legner 1987a,b; 1988a).
Males can change a female\'s oviposition phenotype upon mating, by
transferring an unknown substance (Legner 1987, 1988a,c). Some genes in the
female apparently have the phenotypic plasticity to change expression under
the influence of substances in the male seminal fluid. The intensity of this
response depends on the genetic composition of the male and female. Full
expression occurs in the F1 virgin female (Legner 1987a, 1988a).
The mated female receives a message from the male after mating that expresses
his genome for the presence
or absence of polygenes governing quantitative behavior, such as fecundity.
The discovery of this behavior in M.
raptorellus has opened
questions into the nature of polygenic loci. The ability of the male
substance to switch loci on or off in the female suggests active and inactive
states for such lock. Polygenic loci generally have been thought to be coded
for a fixed kind of expression (Wright 1986).
Greater importance may be placed on liberated males during mass release
strategies that seek seasonally to accelerate and increase the magnitude of
parasitism, because it may be possible to convey directly to unmated females
already resident in the environment certain desirable strain characteristics.
In the process of hybridization, wary genes may serve to quicken the pace of
evolution by allowing natural selection to begin to act in the parental
generation (Legner 1987, 1988a, 1988c).
Tabanidae, or horseflies,
although widespread and on occasion serious pests and vectors of disease to
livestock, have not received much attention. Only one successful inundative
release of the egg parasitoid, Phanurus
emersoni Girault, has been
recorded (Parman 1928). Apparently, this effort was precipitated by a severe
outbreak of anthrax at the time and since this disease diminished and other
control tactics are available, interest in their biological control has not
been fostered.
Flies associated with cattle droppings, symbovine flies (Povolny 1971), have received the most attention
for biological control over the last two decades. The primary targets for
control have been the bush fly, Musca
vetustissima Walker, the
hornfly, Haematobia irritans (L.), and the facefly,
Musca autumnalis DeGeer (Wallace & Tyndale-Biscoe 1983,
Ridsdill-Smith et al. 1986, Ridsdill-Smith &
Hayles 1987). The primary emphasis of control has been on habitat destruction
through the use of introduced dung-burying scarab beetles. Biological control
through dung destruction has been reviewed by Legner (1986). Although the
introduction of dung beetles has clearly aided agriculture by reducing
operating costs and increasing grazing areas through dung removal, it has not
had a great impact on the densities of flies in any area. As there are no
practical non-biological control methods to reduce fly numbers, and the
addition of more scarabs may actually exacerbate the problem, it is thought
that the most logical direction for research is to intensify world wide
searches for more effective natural enemies, especially predators and
pathogens.
A number of pathogens have been isolated from various species of muscids and
some studies have been conducted evaluating their role as control agents. For
example, the exotoxin of Bacillus
thuringiensis Berliner has
been shown to reduce fly production under certain conditions. However, only a
few of these agents appear to show great promise for manipulative use (Daoust
1983a,b; Mullens 1986, Mullens et
al. 1987a,b,c).
Wasps (yellow jackets) are widespread pests in recreational areas and in
urban environments, yet no extended efforts to control them biologically has
ever been made. However, African
honeybees, or "killer bees" as they frequently are called,
have invaded North America from South America through Mexico. Their first
appearance in south Texas in spring of 1991 was accompanied by an increase in
attacks on humans, and they have since become widespread in California and
Arizona by 1999 (Legner, unpub. data; Taylor 1985). A public health problem
may be expected within a year of the invasion as people become aware of these
bees and succumb to their attacks. However, studies on honeybee behavior at
higher latitudes in South America suggest that the public health threat is
not as great as these bees\' notoriety (Taylor 1985). Nevertheless, mosquito
abatement districts in California will undoubtedly be called upon for
information about how to deal with the bees and perhaps to exterminate feral
colonies.
Most of the characteristics that distinguish African bees from European bees,
such as aggressiveness, early-day mating times, degrees of pollen and honey hoarding,
etc. are thought to be quantitative and, therefore, under the control of
polygenic systems. Unfortunately, because of difficulties inherent in
studying quantitative traits in honeybees, knowledge of this phase of their
genetics is scant. In fact Taylor (1985) acknowledge that there is an overall
limited understanding of honeybee genetics. Thus, we really cannot predict
what will occur following hybridization of African and European races because
practically all opinions are derived from their behavior in South America
(Kerr et al. 1982, McDonnell 1984, Rinderer et al. 1982, 1984; Taylor 1985).
Perhaps some indications can be obtained from other groups of Hymenoptera.
A great deal of information about hymenopteran quantitative inheritances has
been gathered recently from parasitic wasps in the genus Muscidifurax that attack synanthropic Diptera, as
previously discussed. If similar systems prevail in honeybees, greater
importance could be placed on drones because it may be possible for African or
European drones to convey directly to unmated queens of either race some of
their own racial characteristics. The rapid Africanization of European bee
colonies in South and Central America could be explained partly by this
process, although early-day mating of African drones has been considered
primarily responsible (Taylor 1985). It is admittedly presumptuous at this
time to infer similarities in the genetics of genera Apis and Muscidifurax,
and the presence of wary genes
in both. Some speculation seems justified where similarities might exist,
however, especially as there is general agreement that permanent control of
Africanized bees will probably involve genetic manipulation and mating
biology (The Calif. Bee Times 1988). If present, wary genes could offer a
means to the abatement of this potentially severe public health pest.
However, the possible occurrence of similar hybridization events in
honeybees, as has been observed in Muscidifurax,
would dictate extreme caution in setting into motion any processes that might
lead to the formation of new races. Available means for identifying
hybridized colonies and extirpating Africanized queens (Page & Erickson
1985, Taylor 1985) are tedious and imperfect. With the understanding that
hybridization events and wary genes of the kind found in Muscidifurax have yet to be substantiated in Apis, the following suggestions
for African bee abatement are tentative.
Deployment of Wary
Genes in
Abatement.--Wary genes could be used to induce in queen
bees immediate behavioral changes such as a reduced dispersal tendency,
greater susceptibility to winter cold, lower fecundity, or even a preference
for subsequent matings to occur in the afternoon when European drones are
most active.
Africanized queens that mate with different races of European drones might
exhibit immediate postmating depression in some cases, as was reportedly in
some species of Muscidifurax
(Legner 1988d). However, the offspring of crosses between African queens and
certain races of European drones might be expected to show heterosis,
expressed as increased fecundity and stamina, while other crosses involving
different races of European bees might produce a negative effect. Crosses
between hybrid queens and hybrid males could result in superactive queens
after mating, following by even more highly active progeny, as was observed M. raptorellus (Legner, unpub. data).
Selection favoring the superactive hybrids would tend to guarantee the
survival of both parental races and a continuous formation of hybrid bees, as
has been suggested for Muscidifurax
(Legner 1988b). Such a process could direct events leading to the relatively
rapid evolution of a new race. A superiorly adapted race might displace
Africanized bees and prevail in the area. Of course this race also would have
to display desirable characteristics of honey production, pollination, and
nonaggression to be acceptable.
Mating European queens with races of drones from feral northern European
populations might causae such queens to acquire increased winter tolerance
and give rise to hybrids that have even greater tolerances. On the other
hand, having drones available that possess a reduced winter tolerance could
increase winter kill.
The selection of appropriate populations for intraspecific crosses is
critical to avoid detrimental outcomes from negative heterosis, or hybrid
dysgenesis, as well as undesirable positive heterotic behavior, such as an
increased aggressiveness. Preintroduction assessments are essential to reveal
such tendencies (Legner 1988b).
The introduction of alien alleles into a population by hybridization
utilizing naturally evolved parental populations would probably be less risky
than introducing genetically engineered ones where no natural selection has
acted priorly. Researchers working to inject laboratory engineered products
into natural populations should consider what kind of behavior will be
demonstrated once heterosis has had a chance to act. Unless the engineered
populations can be completely isolated reproductively from resident wild
populations, there is considerable risk involved.
A lot of other possibilities could be imagined. However, the first step
should involve a more thorough understanding of honeybee genetics, and
whether or not enough similarity exists with known hymenopteran systems to
derive safe and viable strategies. Certain aspects of genetics are as yet
unclear in Hymenoptera, which was demonstrated with the discovery of paternal
influences in males (Legner 1989d). However, there is a clear rationale for
pre-introduction assessments as presently advocated for parasitic Hymenoptera
(Coppel & Mertins 177, Legner 1986a, 1988b).
Berg (1975), Bay et al. (1976), Garcia & Huffaker
(1979) and McCullough (1981) have reviewed developments in biological control
of mainly freshwater snails, especially as they relate to the transmission of
trematode parasites of humans and their domestic animals. Discussion here is
restricted to some pertinent points of those reviews and to some developments
that have occurred since their completion. Predators.--Many
general predators, including species of fish, frogs, birds and certain
aquatic insects, consume fresh water snails. Domestic ducks have been used
with some success in China by herding them through rice fields to forage for
food. However, of all these general predators, only certain tilapine fishes
have been given research consideration as possible biological control agents.
Fish in the genera Oreochromis,
Sarotherodon, and Tilapia feed directly on snails
during various stages of their life cycle. This occurs primarily because the
feeding behavior of these fishes is frequently in the vegetation or detrital
zone that is also utilized for feeding by snails. Larger adult species of Oreochromis and Sarotherodon feed directly on
adult snails, but this predation has not been observed for Tilapia adults. Tilapia only consume snails
incidentally during their normal foraging on plant materials (Roberts &
Sampson 1987).
Possibly the greatest impact of these fish on snail populations is through
competition for resources. Roberts & Sampson (1987) stated that generally
Tilapia compete directly
with the snails that feed on higher plants while Oreochromis competes with snails that feed on algae. In
addition to competition for food, these fish alter the habitat and therefore
have a disruptive effect on the snails\' life cycle.
Certain species of sciomyzid flies
are probably the most host specific predators of snails. Several hundred
species have been described, the larvae of which depend on mollusks for food.
Of six species that were studied for biological control, two successful
introductions have been recorded and those were the release of Sepedon macropus Walker and Sepedon
sauteri Hendel into Hawaii
to control the intermediate host of the giant liver fluke of cattle. Success
of these releases was apparently shown by a reduction in liver infections at
slaughter houses (Bay et al. 1976, Garcia & Huffaker
1979). Berg (1973) emphasized that because there are several hundred species
in this family with a wide range of biological attributes, they offer great
opportunity for matching a certain sciomyzid with the appropriate ecotype
snail. Unfortunately the scope of opportunities for use of these flies for
snail control has not been given the attention it deserves. Antagonists.--Another
approach for control of snails has been through interspecific competition. The
large predatory snail Marisa cornuarietis L., has been
evaluated rather extensively Puerto Rico and has been shown to be effective
for control of Biomphalaria glabrata Say, the
intermediate host of human schistosomiasis, in certain habitats, especially ponds.
Suppression of B. glabrata by Marisa is
primarily due to competitive feeding and to incidental predation on the
immature stages of this snail (McCullough 1981).
In Africa M. cornuarietis eliminates three species of
pulmonate snails (Biomphalaria
sp., Bulinis sp., and Lymnaea sp.) in a water
impoundment in northern Tanzania. Prior to release of M. cornuarietis,
three pulmonate species in addition to a melaniid snail, Melanoides sp., existed in large thriving populations. Two
years after the introduction only M.
cornuarietis and the
melaniid snail remained, the latter in population densities similar to
preintroduction levels (Nguma et
al. 1982). No adverse
environmental effect was recorded in this situation; however, the authors
stressed that a careful examination of potential environmental risks should
be made before introduction to a new area.
Another competitor snail, Helisoma
duryi (Wetherby), has shown
promise for the control of B.
glabrata. Christie et
al. (1981)
working with the ram\'s horn snail, H.
duryi, showed that it
controlled B. glabrata in artificial outdoor
drains on the Caribbean island of St. Lucia. The elimination of B. glabrata may have been due to inhibition of reproduction
by adults and possibly to increased mortality of immature snails. The time
required for elimination was related to environmental temperature and the
number of H. duryi initially released. In
Africa Madsen (1983) surveyed H.
duryi as an introduced
species in an irrigation scheme in northern Tanzania and found it restricted
to just a few drains 10 years after it had been established in the area. He
noted that its failure to spread may have been related to the routine
molluscacide applications to the irrigation canal system.
Moens (1980, 1982) achieved successful biological control of Lymnaea truncatula Muller, an intermediate host of the trematode, Fasciola hepatica L. in watercress in Belgium, with the predatory
snail, Zonitoides nitidus Muller. Predation was
related to temperature, soil moisture and cover.
It is obvious that the role of biological control of snails as intermediate
hosts of human diseases is limited. As McCullough (1981) pointed out, it will
be restricted to specific situations and will rarely, if at all, have
widespread applicability. In addition it will play only a supportive role in
almost all geographic areas where schistosomiasis and other snail transmitted
diseases exist. However, this does not mean that biological control is not
important. Indeed, any method that reduces transmission of a disease in a
self-sustaining fashion is of major benefit. References (please refer to following articles for specific
references): Garcia, R. & E. F. Legner. 1999. Biological Control of Medical and Veterinary Pests. In:. Bellows, Jr. & T. W.
Fisher (eds) 1999. Handbook of Biological Control: Principles
and Applications. Academic Press, San Diego, CA Legner, E. F. 1995. Biological Control of Diptera of Medical and Veterinary Importance.
J. Vector Ecology 20(1): 61 p. |