FILE: <ch-133.htm> GENERAL INDEX [Navigate to MAIN MENU ]
|
WALNUT HUSK FLY Rhagoletis completa Cresson
(Insecta: Diptera: Tephritidae) (Contact) Erich F. Legner and Richard
D. Goeden University of California
Riverside CLICK on Photo and Tables to
enlarge & search for Subject Matter with Ctrl/F. GO TO ALL: Bio-Control Cases Dr. S. H.
Berlocher of Southern Illinois University informed us of a high incidence of
larval parasitism of walnut husk fly on wild Juglans microcarpa
Berlandier by an opiine braconid during a brief period of September in its
native rage in the Davis Mountains of western Texas in 1974. Dr. Berlocher found that husk fly larvae could only be obtained from J.
microcarpa only during a short period from mid August and early
September. Most walnut fruit drops from trees by the second week of September
and the larvae immediately exit from the fallen fruit to enter the ground to
pupariate. This information prompted field collections of R. completa
larvae and a measurement of parasitoid activity throughout the Davis Mountain
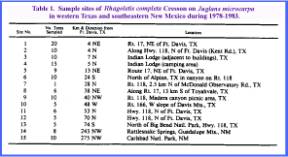
area during early September of 1978 through 1983. Surveys
actually were conducted on J. microcarpa from near Carlsbad, New
Mexico throughout the Davis Mountains of Texas and south to the northern
boundary of Big Bend National Park (Table
1). This region lies within the
Chihuahua Desert between the Rio Grande and Pecos Rivers and has many prominent
mountains, which usually support a grassland climax vegetation (Warnock
1970). Juglans microcarpa is
a common tree along arroyos at elevations between 1200 and 1600 m. Table 1 (CLICK to enlarge) METHODS
DEPLOYED IN SAMPLING AND
APPRAISAL Samples of
whole, blackened infested walnuts were taken from trees during the final week
of August in 1978, 1980, 1981 and 1983.
However, all sites could not be sampled each year because the fruit
had fallen prior to our arrival.
Fruit fall depended on variable weather conditions such as wind and
rainfall. An estimate of larval
density per walnut was made in 1980 by sampling 300 walnuts at random per
tree. These were then placed on 4 cm
of local soil in polyethylene buckets for 5 days to allow larvae to exit the
nuts and pupariate in the soil.
Puparia were then carefully and with minimum abrasion sifted from the
soil and placed in 12 dram screened polystyrene containers. The caged puparia were transported to the
quarantine at the University of California in Riverside, where they were
stored in refrigerators at 3◦+1◦
C and 55% RH for 6 months. Samples
were then incubated at 25◦ C, 55% RH and a 14:10 h L:D photoperiod to
allow emergence of adult flies and parasitoids. Unemerged puparia were refrigerated for another 6 months
beginning the following September, followed by another period of incubation
to promote additional emergence. A
third such refrigeration/incubation cycle also was performed. Identification of Rhagoletis completa
was verified by comparing adult specimens with those identified for the
Department of Entomology by F. L. Blanc and R. H. Foote and by referring to
the descriptions in Boyce (1934) and Michelbacher and Ortega (1958). Trybliographa sp. was identified by
Gordon Gordh and Biosteres sublaevis by R. A. Wharton from
material we provided Dr. Kenneth Hagen of UC Berkeley, some of which probably
were included in Wharton's type series.
We identified subsequent collections of sublaevis by reference
to Wharton and Marsh (1978). For
statistical analyses the larval walnut husk fly densities per walnut at the
time of initial field sampling were compared to the final densities after
parasitization and other mortality factors had acted. These density differences measured
parasitoid response to varying host densities in the field and determined
whether such response was regulative, i.e. an increasing proportion of hosts
were parasitized at higher host densities.
First, the initial larval density in 300 sampled walnut fruit per tree
was compared to the parasitized host density using a bivariate correlation
analysis. Secondly, host regulative
response was analyzed by correlating the log 10 (initial density + 1.0) with
the difference between lo1 10 (initial density + 1.0 and the log 10 (final
density + 1.0), i.e., the "killing power or "k-value" of
Varley et al (1974). Correlation
coefficients were all tested at P < 0.05. Determination of a parasitoid's activity from incubation and
emergence data in the laboratory, however, may underestimate its actual
impact. Some hosts may be killed by
the probing and oviposition of parasitoids thus not giving rise to adult
parasitoids as suspected previously for other insects (Legner 1979, Legner
and Silveira-Guido 1983). Also,
although considerable care was taken to provide a natural situation for
pupariation, and handling was done as little as possible, some developmental
anomalies may have occurred during the pupal stage. These may result in adult emergence failures. The U. S.
Dept. of Agriculture in Bethesda, Maryland authorized Biosteres sublaevis
introduction in California wild and cultivated walnuts. Before finding suitable insecticide-free
orchards of Juglans regia wild species of husk fly infested Juglans
in California served as the plant host for cultures of the parasitoids. After
exposure to parasitoids in the laboratory these wild California Juglans
were periodically distributed after 1983 into various undisturbed natural
host habitats of Ventura, Los Angeles and Riverside Counties from where they
had originally been collected. One
insecticide free organically managed orchard of Juglans regia in Ventura County also received Rhagoletis completa
liberation in this manner. FINDINGS
AND DISCUSSION Adults of only Rhagoletis completa emerged from the
wild walnuts collected in the Texas and New Mexico study areas, although Rhagoletis
juglandis (Musebeck) is known to occur in the northwestern portions
(sites 14 and 15). The first refrigeration/incubation cycle stimulated
>95% of the total emergence of host flies and parasitoids in every sample
(Table 2). Biosteres sublaevis was the most prominent parasitoid species
reared. Trybliographa sp.
occurred at much lower frequencies, and always in conjunction with the former
species. Parasitism was widespread
throughout the sample area and varied considerably from year to year at any
given site (Table 2). There may have been a trend toward higher
parasitism in areas protected from the fill impact of storms from the north
by rises of the Davis and Guadalupe Mountain ranges, whereas in the more
open, northerly exposed and windswept areas, e.g. 10, 11 and 13 (Table 1 and Table 2), parasitism was comparatively
lower. Site 12 was sheltered by the
northernmost foothills of the Davis Mountains and showed relatively high
parasitism. Table 2 (CLICK to enlarge) High mortality in puparia also was recorded at all
collection sites (Table 2). This mortality was not correlated with
intensity of parasitoid emergence (r - -0.186, 41 df), and probably was
caused by combinations of handling, parasitoid probing and aborted
parasitism. There was also a significant correlation between the
initial within walnut larval density and the final adult fly emergence
density in 1980 (r - 0.777, 14 df). A
subsequent k-value analysis (Varley et al. 1974) also showed a significant
correlation (0.494, 14 df). This
indicated that fly mortality from all natural factors combined occurred in
greater proportions at relatively higher initial larval densities. However, it cannot be ascertained whether
parasitism was the main regulative factor because there was no significant
correlation between the initial host larval density and the Biosteres density
(r = 0.308, 14 df). Data pertaining
to inter-tree and inter-walnut density might give further clues to the
regulative ability of the parasitoids.
Nevertheless, problems associated with measuring the full impact of
any parasitoid on its host in the wild as reviewed recently (Legner 1983,
Legner and Silveira-Guido 1983) obviously also contributed to our inability
to access this natural parasitism more fully.
The wide distribution and
high intensity of walnut husk fly larval parasitization by Biosteres
sublaevis in the surveyed areas has prompted an effort to introduce this
species into California from Texas for biological control. LITERATURE
CITED CLICK Highlighted for greater
detail
Berlocher, S. H. 1976. The genetics of
speciation in Rhagoletis (Diptera: Tephritidae). Ph.D. Thesis, Univ. of Texas, Austin. 203
p.
Boyce, A. M. 1934. Bionomics of the walnut husk fly, Rhagoletis
completa. Hilgardia 8: 363-579.
Legner,
E. F. 1979. The relationship between host destruction
and parasite reproductive potential in Muscidifurax raptor, M. zaraptor and Spalangia
endius (Chalcidoidea: Pteromalidae).
Entomophaga 24: 145-152. Legner, E. F.
1983. Requirements for appraisal of the role of
parasitic insects in the natural control of synanthropic Diptera.
Proc. Calif. Mosq. & Vector Control Assoc., Inc. 51: 97-98 Legner, E. F. & R. D. Goeden. 1987. Larval parasitism of Rhagoletis completa (Diptera:
Tephritidae) on Juglans microcarpa (Juglandaceae) in western Texas
and southeastern New Mexico. Proc. Entomol. Soc. Wash.
89(4): 739-743.
Legner,
E. F. & A. Silveira-Guido.
1983. Establishment of Goniozus
emigratus and Goniozus legneri (Hym.: Bethylidae) on navel orangeworm, Amyelois transitella
(Lep.: Phycitidae) in California and biological control potential. Entomophaga 28: 97-106. Michelbacher, A. E. & J. C.
Ortega. 1958. A technical study of insects and related
pests attacking walnuts. Calif. Agric. Exp. Stn. Bull. 764. 86 p.
Varley, G. C., G. R. Gradwell & M. P. Hassell. 1974.
Insect Population Ecology, an Analytical Approach. University of California Press, Berkeley & Los
Angeles. 212 p. Warnock, B. H. 1970. Wildflowers of the Big Bend Country, Texas. Sul Ross State University, Alpine, Texas. 157 p.
Wharton, R. A. & P. M. Marsh.
1978. New world Opiinae
(Hymenoptera: Braconidae) parasitic on Tephritidae (Diptera). J.
Wash. Acad. Sci. 68: 147=167. |