|
Laboratory Materials |
|
POLYTENE CHROMOSOMES & CHROMOSOME
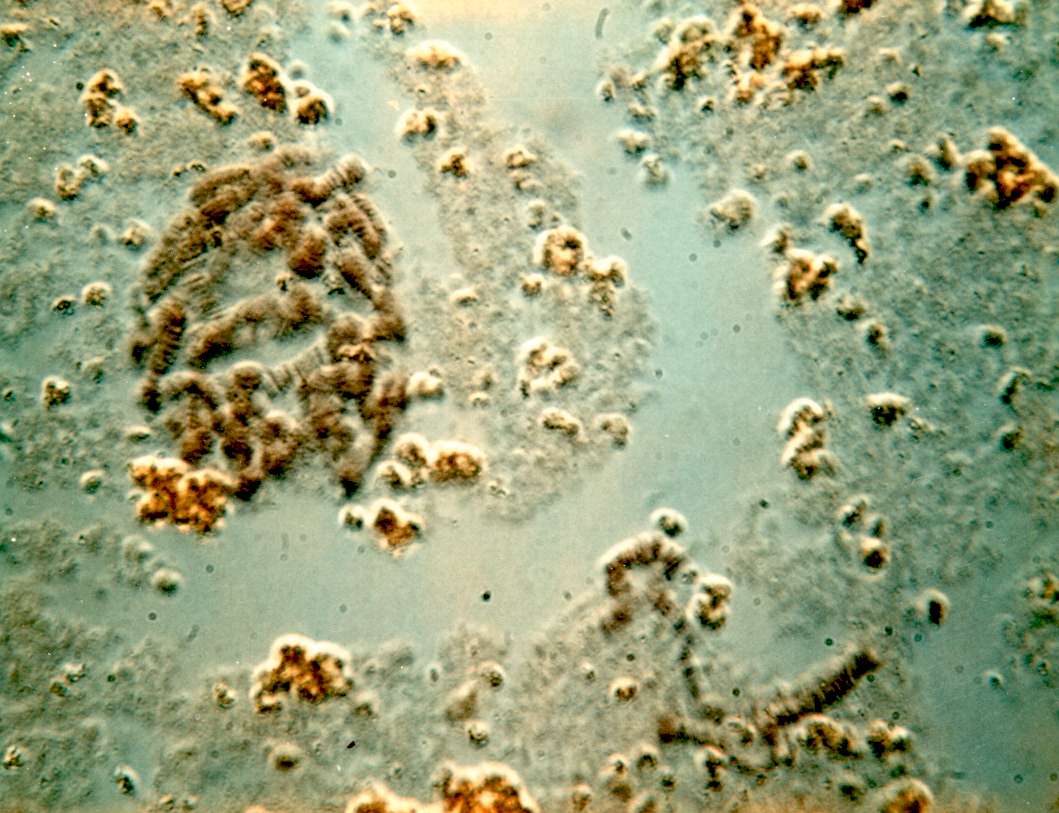
PUFFING Objective: To stain and identify the polytene chromosomes of the salivary glands of Drosophila melanogaster. To induce puffing, which is a visual indicator of RNA transcription. Introduction: Many larval and some adult tissues of insects in the family Diptera are characterized by nuclei with giant chromosomes. These chromosomes develop by multiple replications of the chromosomes within each cell during development. Each nucleus will contain hundreds of copies of each chromosome. Cells are considered polyploid if they have more than two copies of each chromosome. If the chromosomes align perfectly forming large cables of chromosomes they are polytene. In Drosophila melanogaster, chromosomes of the larval salivary gland contain about 1024 copies of the DNA, or ten doublings from the normal 2n condition, of each of the three chromosomes. Each gene is exactly aligned with its homologs on the other 1023 copies. The pattern of condensed regions (heterochromatin), and transcribed regions (euchromatin) gives a series of about 5000 light and dark bands when the chromosomes are stained with orcein. The banding patterns of the chromosomes show significant phylogenetic and ontogenetic stability. Genetic maps relate these bands to their functions. In general, the DNA in each band codes for a single function, although there are exceptions to this observation. Drosophila has given us substantial insight into DNA function and gene organization. In March 2000, the full genome map of D. melanogaster was published in the journal Science. At certain times during development, some bands undergo a reversible modification to form structures known as "puffs." Puffs are localized expansions of the polytene chromosome structures. Puffs are sites of synthesis of RNA and result from the activation of the gene (or genes) contained within a particular band. This means that changes in gene activity can be observed microscopically as changes in polytene chromosome puffing pattern. The size of the puff reflects the number of copies of that gene that are being transcribed - larger puffs have a larger percentage of the 1024 chromatids being simultaneously copied into mRNA. The specific RNAs are translated into a set of polypeptides Puffing patterns that occur during normal development can sometimes be induced by experimental treatments. Changes in the patterns of puffing occur as a direct consequence of changes in the concentration of the insect's growth and molting hormone, ecdysone. Initial changes in puffing can be seen in salivary glands exposed to ecdysone for less than 15 minutes. The pattern of gene activation starts with new transcription of a few "early" genes whose products in turn regulate a set of "late" genes. This regulation can include increased or decreased synthesis as well as initiation or termination of transcription. Increased or decreased transcription can also result from a wide variety of environmental factors including a brief heat shock. In 1962, heat shock was found to induce a unique set of puffs and the tranlation of the heat shock polypeptides or "hsp's". When Drosophila larvae, or their excised tissues, are subjected to a brief heat shock (for example, 40 min. at 37o C, the normal culture temperature being 25o C), puffs are induced at a few specific sites. In D. melanogaster there are nine heat inducible puffs, in a related species, D. hydei, there are six. The induction of the puffs by the heat shock is very rapid; it occurs within one minute of the temperature increase though the puffs continue to increase in size for some 30 to 40 min (at 37o C) before regressing. The maximum sizes of the induced puffs are a function of the severity of the temperature shock, at least until lethal temperatures are met. The induction requires RNA, but not protein synthesis. However, in the absence of protein synthesis the induced puffs fail to regress unless the temperature is returned to normal. Prolonged temperature shock (e.g., more than 1 hour) results in additional changes in puffing activity; all other puffs active at the time the temperature shock commenced regress. The heat shock response of specific RNA and protein synthesis occurs in all tissues of the fly and has been observed in permanent tissue culture cell lines. In this experiment, you will dissect out the salivary glands of Materials:
Drosophila melanogaster
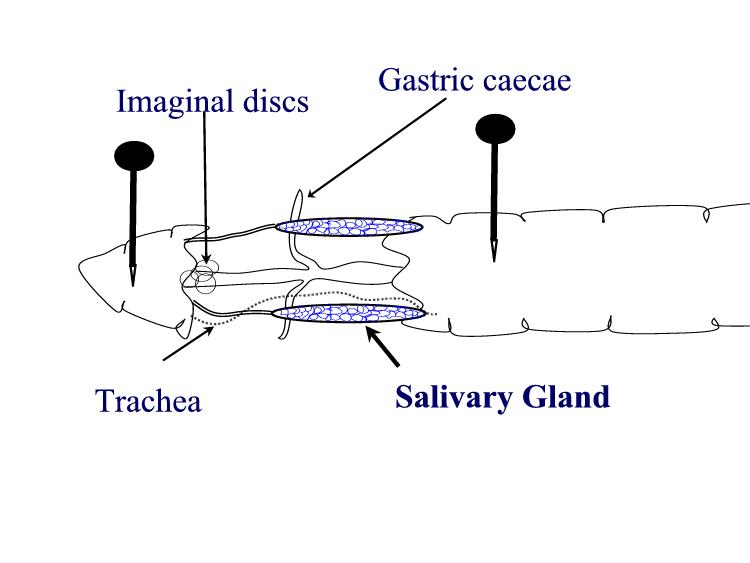
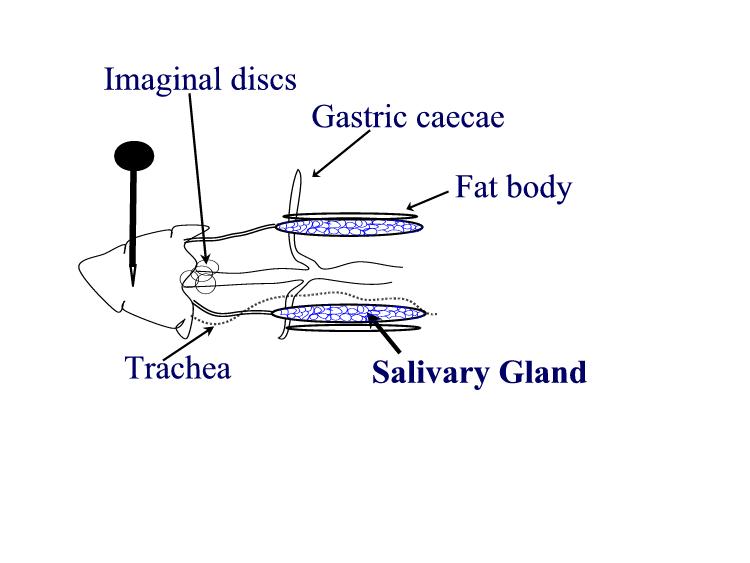
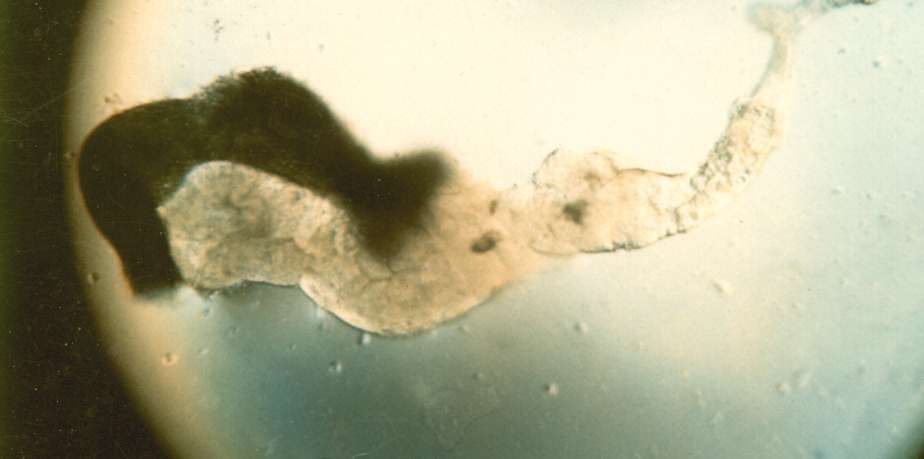
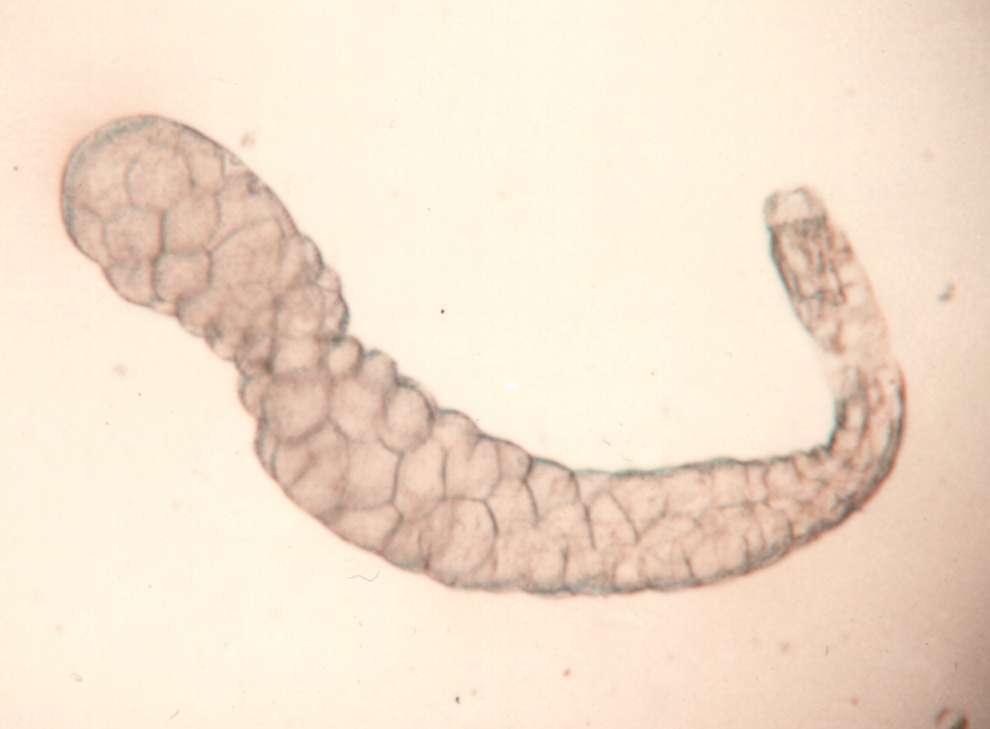
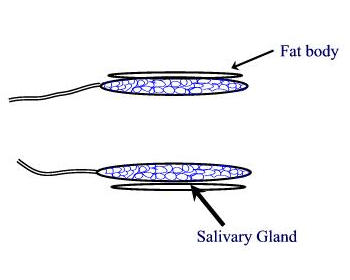
larvae Procedures: Use mature third instar larvae - the fattest crawling larvae in the small petri dishes. If the larvae have started to form the puparium, the fat body quickly disappears to make room for the adult salivary gland. 1. Adjust your dissecting microscope so it has a dark background or dark field where the light is coming from an angle so you can see the tissues clearly. 2. Dissect out the salivary glands
in 45% acetic acid. Glands should remain in acetic acid for relatively
short periods of time. Do not allow the glands to dry.
3. Carefully remove most of the drop of acetic acid by tilting the slide and removing the drop with a kimwipe. Keep the kimwipe away from the gland itself. Add one drop of 1 N HCl for 30 seconds then carefully remove. 4. Quickly rinse the gland with lacto-acetic acid to prevent precipitation of stain. 5. Add one drop of stain and cover slide with half of a petri dish. Wait 15 minutes. 6. Rinse stained glands with lacto-acetic acid. Place cover slip over gland and put it between layers of paper toweling. Press firmly on the cover slip, taking care that it does not slip. You may need to prepare two or three slides before you master the skill of spreading the chromosome arms without tearing the chromosomes. 7. Dissect a second gland and repeat staining in case the first is not successful. 8. Examine the chromosomes. Adjust your microscope for Kohler illumination and look for large pinkish nuclear regions. If you look carefully along the length of each chromosome, you may see puffs, genes that are being transcribed.
9. If you have a copy of the Drosophila chromosome map handy, you can attempt to identify chromosomes 1, 2, 3, and 4.
Solutions:
Lacto-acetic acid: 85% lactic acid: 60%
acetic acid: deionized water 1:1:1
Modified from a laboratory procedure by Jeanette Holden, formerly of Queen's University, Kingston, Ontario.
|
 |
Click on the picture to go to Dr. Miller's Lab Web Page. |
|
More Topics on the Wing |
|
|
|
About Us |
| Click on Picture to go to the link |
Page Designed by
Harald Baella. Last updated
01-25-05
Copyright © 2003-05 Miller Web Design.